|
 |
- Search
| Obstet Gynecol Sci > Volume 58(6); 2015 > Article |
Abstract
Objective
The purpose of the study was to examine the relationship between the parameter representing ovarian reserve and the fetal aneuploidy in early spontaneous miscarriage.
Methods
A multicenter retrospective cohort study was performed in patients who were diagnosed with early pregnancy loss (≤13 gestational weeks) and examined for fetal karyotype at the CHA Gangnam Medical Center, CHA Bundang Medical Center, and CHA Gumi Medical Center between January 2011 and December 2012. Karyotyping was performed by the Genetic Laboratory of the Fertility Center of CHA Gangnam Medical Center. Medical records were reviewed for demographics, karyotype analysis and hormonal assay of ovarian reserve including antimullerian hormone (AMH) and follicle stimulating hormone. Statistical analysis was performed using SPSS software.
Results
A total 462 patients were included in this study. The mean age of the patients was 35.31±4.12 years and the mean AMH level was 3.88±3.50 ng/mL (n=195). Two hundred eleven conceptuses (45.7%) of patients showed the euploid and 251 (54.3%) showed the aneuploid. There are significant differences in maternal age, AMH and gestational age between fetal euploid and aneuploid groups (34.46±4.35 vs. 36.04±3.78 years, P<0.001; 4.60±3.86 vs. 3.43±3.18 ng/mL, P=0.022; 7.67±1.54 vs. 8.27±1.46 weeks, P<0.001, respectively). Multivariate analysis revealed that low AMH level and early gestational age were maternal age-independent markers for fetal aneuploid (P<0.001 and P=0.045, respectively).
Early pregnancy loss is the loss of a pregnancy before 20 gestational weeks. Although the exact mechanisms responsible for abortion are not always clear in the first trimester, fetal factors including aneuploid are the predominant etiology and account for 80% to 90% of early miscarriage. Among fetal factor in terms of the first trimester, approximately half of miscarriages are embryonic causes and half of those is due to fetal chromosomal aberrations.
Fetal aneuploidy seems to occur as a result of increased chromosomal nondisjunction during the long arrest period in meiosis I, before ovulation [1,2,3,4]. There is well-known association between fetal aneuploidy and the maternal chronologic aging. Maternal chronologic aging is correlated to decreased ovarian reserve which is predisposed to a greater risk for fetal aneuploidy [5,6]. Based on such an association between maternal ovarian reserve and fetal aneuploidy, multiple predictive markers in terms of ovarian reserve including follicle stimulating hormone (FSH) and antimullerian hormone (AMH) level have been studied to predict fetal aneuploidy [7,8].
AMH, synthesized by human granulosa cells in the ovary, has exhibited significant promise as a potential marker of ovarian reserve [2,9,10,11]. Serum AMH levels decline with age. Several studies have shown that AMH is a better predictor of ovarian reserve than age [12,13]. It offers several advantages over other traditional markers to predict ovarian reserve. Oocyte quality as well as oocyte quantity affect embryo quality and seem to be associated with aging. AMH is postulated to be associated with both ovarian reserve and function. Another advantage of AMH is its stability. AMH level is constant throughout the menstrual cycle and is not influenced by gonadotropin-releasing hormone agonists, pregnancy, or oral contraceptives [11,14,15,16,17].
Despite of such an advantages and expected close association with ovarian reserves of AMH, limited number of the study regarding the association between ovarian reserve and fetal aneuploidy in early spontaneous miscarriage was reported.
Therefore, the objective of the present study was to examine the direct relationship between parameters that represent ovarian reserve including AMH and the incidence of fetal aneuploidy in early spontaneous miscarriage.
A multicenter retrospective study was performed with patients who were diagnosed with the first trimester pregnancy loss between January 2011 and December 2012 in CHA Gangnam Medical Center, CHA Bundang Medical Center, and CHA Gumi Medical Center. This study was approved by the institutional review board of CHA Gangnam Medical Center, CHA University, Korea. Only singletone pregnancies were included in the study. A dilatation and curettage was offered to all patients after the diagnosis of early pregnancy loss before 13 gestational weeks. Pregnancy loss was confirmed by repeat ultrasound examination. Conceptus which had been spontaneously expelled also processed karyotype testing.
Karyotyping was conducted by the Genetic Laboratory of the Fertility Center of CHA Gangnam Medical Center. Karyotype analysis was performed by using classical karyotype cytogenetics (standard tissue culture) or multiplex ligation-dependent probe amplification (MLPA) which remained as consistent techniques over the 10-year period.
Patient characteristics, such as maternal age, gestational age, parity, reproductive history, method of conception, maternal body mass index (BMI), AMH level and FSH levels were reviewed. As conceptional method, natural pregnancy was defined when conceived naturally or with method of intrauterine insemination (IUI) and timed intercourse without controlled ovarian hyperstimulation (COH). In case of COH, the other categories were divided as in vitro fertilization (IFV) embryo transfer (ET), intracytoplasmic sperm injection (ICSI), IVF-Freezing ET and IUI according to the procedures.
Each patient's serum AMH and FSH level, serving as a marker of ovarian reserve, was measured at especially infertility clinic. The blood sample for hormone assay were obtained on menstrual cycle day 3 to 5. Serum AMH was quantified with AMH Gen ll ELISA (Beckman-Coulter, Inc., Brea, CA, USA). This assay is standard monoclonal antibody sandwich enzyme immunoassay which is specific for AMH and do not exhibit any significant cross-reactivity with related molecules. All AMH level of the patients were analyzed by this sole assay, which minimize the error caused by using diverse assay. If sample assay was not be completed within 48 hours, the samples were freezed at -20℃.
Statistical analyses were performed using the IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA). Chi-square test and Student's t-test were used for the calculation of significance. The descriptive data were expressed as the mean±standard deviation. Univariate and logistic regression analyses were performed to evaluate the association between parameters of ovarian reserve and fetal aneuploidy. For multivariate analysis, advanced maternal age was defined with patient age (≥35 or <35 years) because increased rates of fetal aneuploidy have been documented in this age group. A P-value of <0.05 was considered statistically significant.
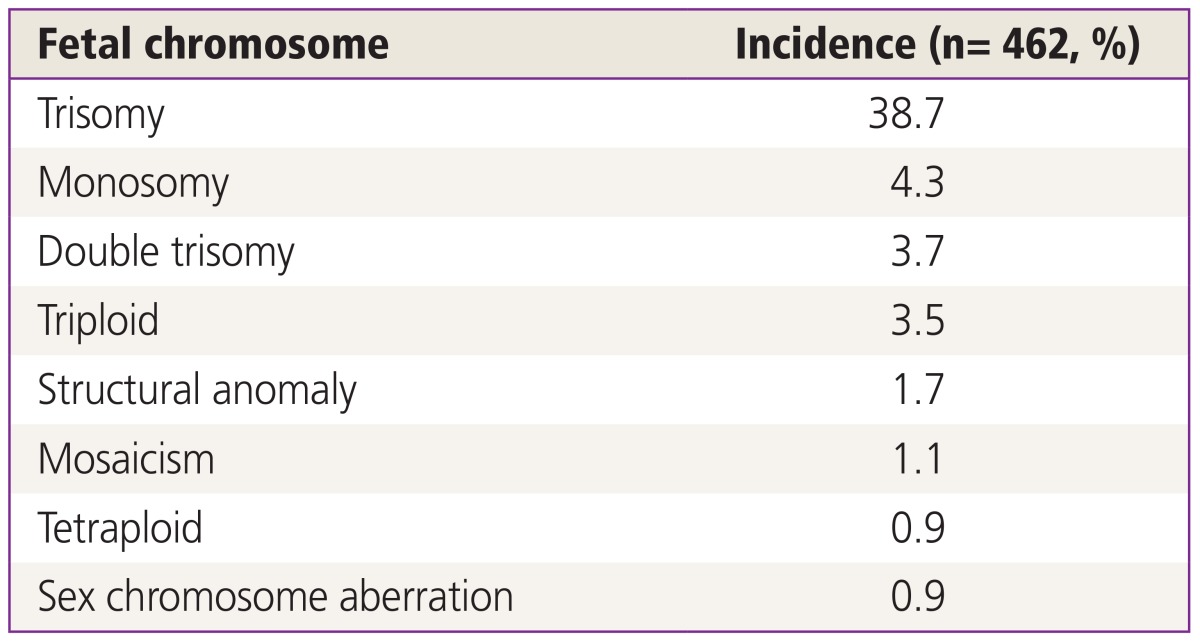
Total 462 patients were enrolled for the study. The mean age of the women was 35.31±4.12 years and the mean BMI was 21.05 kg/m2. A total of 195 patients were tested for AMH level, mean level of which was 3.88±3.50 ng/mL. A total of 264 patients were tested for FSH. The mean level of FSH was 8.53±4.96 mIU/mL. Mean gestational age when the patient was diagnosed with early pregnancy loss was 8.0±1.52 weeks. The number of subjects diagnosed by classical cytogenetic karyotype was 413 (89.4%). Forty-nine (10.6%) subjects were diagnosed by MLPA. Among 462 patients, 211 conceptuses (45.7%) of patients showed the euploid and 251 conceptuses (54.3%) showed the aneuploid. The number of patients who were pregnant naturally was 128 (27.7%) and number of patients who were conceived by timed intercourse was 61 (13.2%). One hundred twenty one patients underwent IVF-ET (26.2%) and 73 received ICSI (15.8%). The most common abnormal karyotype encountered was autosomal trisomy followed by monosomy and double trisomy (Table 1).
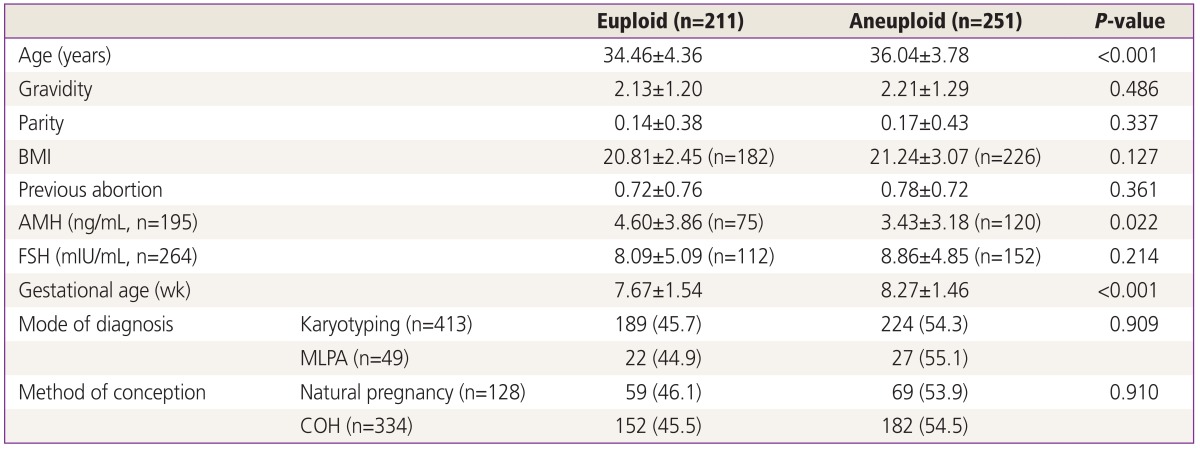
Table 2 compares patient demographics including AMH, FSH, and gestational age between euploid and aneuploid group. The euploid group was younger than aneuploid group (34.46±4.36 vs. 36.04±3.78, P<0.001, respectively). There was no difference in gravidity, parity, BMI and previous abortion history. AMH level is significantly low in fetal aneuploid group than fetal euploid group (3.43±3.18 vs. 4.60±3.86, P=0.022). However, there was no significant difference in FSH level between euploid and aneuploid group (8.09±5.09 vs. 8.86±4.85, P=0.214). Early pregnancy loss was diagnosed earlier in aneuploid group than euploid group (7.67±1.54 vs. 8.27±1.46, P<0.001). By classic karyotype cytogenetics methods, 45.7% and 54.3% karyotype of patients was confirmed as euploid and aneuploid, respectively. By MLPA, 44.9% and 55.1% karyotype of patients was confirmed as euploid and aneuploid, respectively. There was no difference according to mode of karyotyping. In natural pregnancy group, 46.1% showed euploid and 53.9% was aneuploid. In subjects who were pregnant by COH, 45.5% and 54.4% of patients showed euploid and aneuploid, respectively. COH did not affect fetal karyotyping (P=0.910).
Univariate analysis revealed that maternal age, gestational weeks at abortion and AMH level were associated with aneuploid of conceptus. With these three markers, we conducted logistic regression analysis to evaluate whether maternal age affects the relationship of AMH and gestational weeks to abnormal karyotyping (Table 3). When advanced maternal age was analyzed as a categorical variable (age ≥35 years), the association between fetal aneuploidy and AMH level and gestational weeks reached statistical significance. AMH level was higher and gestational weeks was significantly lower in aneuploid group regardless of maternal age (P=0.045 and P<0.001, respectively).
The present study was performed to determine whether a serum AMH level could be an independent predicting marker for aneuploid. The result of the study shows that significant age-independent relationship between elevated AMH level and fetal aneuploid. However, basal FSH is not associated with the fetal aneuploid.
Aneuploidy is the most commonly identified chromosome abnormality in humans, occurring in at least 5% of all clinically recognized pregnancies. Despite the high frequency and devastating clinical results of aneuploidy, we know relatively little about factors that modulate and predict the risk of aneuploidy. Although several risk factors except genetic one have been suggested including oral contraceptives, fertility drugs, thyroid antibodies, alcohol consumption, parity and maternal diabetes, none of these have been proved to have any other associations [18,19,20,21]. The only one indisputable factor associated with fetal chromosomal aberrations is advanced maternal age. Chronologic ovarian aging influence diminished ovarian reserve. Various endocrinological markers have been used to assess the ovarian reserve such as AMH and FSH. There are conflicting data regarding whether compromised ovarian reserve markers are associated with an increase in aneuploid pregnancies [22,23,24,25,26].
AMH is the dimeric glycoprotein and the member of the transforming growth factor-β superfamily. In the ovary, AMH is synthesized by granulosa cells only after birth or at the end of fetal life. AMH regulates steroidogenesis of ovary and influences folliculogenesis. Recent studies indicate AMH as an important novel measure of ovarian reserve. AMH appears to offer several potential advantages over other tests of ovarian reserve. The circulating AMH seems to be solely of ovarian origin because one study found AMH to be undetectable 3 to 5 days after bilateral ovariectomy [27]. Moreover AMH may be a unique endocrine parameter of ovarian function, since several studies have demonstrated that, in contrast to sex steroids, gonadotrophins and peptides, AMH serum levels do not significantly change throughout the menstrual cycle [15,28,29,30].
There are limited numbers of the study that have examined the potential of AMH as predictor of fetal aneuploidy. Seifer and Maclaughlin [11] conducted case-control study using stored serum samples which were obtained for antenatal chromosome screening. The study included 25 Down's syndrome sample and 125 unaffected controls. The authors could not find any difference in maternal serum AMH level between Down's syndrome pregnancies and controls. However, the study only focused on trisomy 21 alone and did not take into account the pregnancies that were terminated in the first trimester.
Plante et al. [24] reported that AMH value declined significantly with advancing maternal age and AMH level did not differ between women with an aneuploidy fetus and women with a euploid fetus. The authors concluded that AMH level did not predict fetal aneuploidy. However, only 18 patients carrying an aneuploidy fetus served as cases and serum AMH level was measured by two different methods which could resulted in a bias.
In this study, AMH, as it was related to age, could not be significant predicting factor of fetal aneuploid when multivariate analysis was done by using age as continuous variable. Authors noticed that clinical predicting factor of fetal aneuploid that was usually used to counsel about infertility or normal pregnancy, was whether patient's age is over 35 or not, and above result was obtained by analyzing using categorical variable appointing age 35 as standard. Using a cutoff of 35 years, significant association between elevated AMH and fetal aneuploidy rate was found in the present study (P=0.045). Compared with previous results, our data reported conflicting result. The reason of conflicting result might be due to the differences in study population. Previous study included samples which were obtained from not patients who were diagnosed early pregnancy loss but subjects who were in on-going pregnancy. In our study, the serum was obtained before pregnancy. On the other hand, previous study analyzed AMH level during pregnancy. Lastly, our study focused on early pregnancy loss and the mean gestational weeks at abortion was around 8 gestational weeks. However, in the other studies, the pregnancy was maintained more than 13 gestational weeks. Those differences might results in conflicting result among studies [11,24].
In comparison to previous study, our mean AMH level was relatively high as 3.43 ng/mL. It is thought to be influenced by involving the whole group not excluding 18 polycystic ovary syndrome patients typically showing high level of AMH.
Elevated basal FSH concentration is a known marker for decreased ovarian reserve. There are conflicting results regarding whether basal FSH level may be directly associated with an increase in aneuploid pregnancies. Several studies suggested that elevated basal FSH concentrations were related to aneuploid pregnancies [23,31]. However, recent studies contradict this conclusion. van Montfrans et al. [32] observed no significant effect of basal FSH concentrations on the incidence of early pregnancy loss or abortion of clinically recognized pregnanices in their prospective study. Massie et al. [33] evaluated whether a basal FSH level could be an independent predictor of fetal aneuploidy. The authors performed the study with 177 spontaneous miscarriage samples which included 70 euploid and 107 aneuploid. The result demonstrated that an elevated basal FSH was not associated with an increase in fetal aneuploid. The result of our study does not support the findings of earlier studies. Although FSH level in fetal aneuploid group was slightly elevated than in fetal euploid group, there was no statistically significant difference between euploid and aneuploid groups. When make a conclusion by these comprehensive studies, FSH concentration most probably cannot be used to predict the risk of fetal aneuploid in the pregnant woman.
Both abortion rates and chromosomal anomalies decrease with advancing gestational age by first, second and third trimester. Percentage of chromosomal anomalies of abortus or stillbirth on each trimester are 55%, 35%, 5% respectively. But in the present study consisting of the only first trimester pregnancy, the miscarriage was diagnosed earlier in aneuploid group than euploid group (7.67±1.54 vs. 8.27±1.46, P<0.001). As our knowledge, no previous study was examined focusing on the association between gestational age and chromosomal anomalies in the early pregnancy loss. Our result suggests that another nonnegligible factor such as infection, medical disorders, immunologic factor, inherited thrombophilias and uterine defects might affect the early spontaneous miscarriage besides fetal factors. Especially, abortion due to immunologic factor happens by miscarriage at implantation stage, and it is thought to be aborted at 1st trimester although fetal karyotype is normal.
There are several limitations in the present study. The first is that there are some missing values of AMH level in spite of the fact that AMH is significant factor to predict fetal aneuploidy. Initial purpose of this study, however, was to look over general ovarian reserve markers that affect fetal aneuploidy. Thus, all patients were included in study although there were missing values of AMH. To check whether this missing value is random missing value or not, independent t-test was used in both euploid group and anuploid group to compare patient characteristics between AMH-known patient group and AMH-missed value patient group, and no difference between two patient groups in both euploid and aneuploid group was found. Second we used two different methods, classical karyotyping and MLPA to examine fetal karyotyping. Lastly, study and control groups were heterogenous. Although infertility treatment did not make any differences in the result of fetal karyotyping, more than half of the subjects were received controlled ovarian hyperstimulation followed by IVF-ET, ICSI and IUI.
Our study offers several strengths compared to previous reports. The Large sample size in this study relative to others increases the reliability of our findings. We definitely identified fetal chromosome by classical karyotyping which was used for precise results.
We tried to make the cutoff point of AMH level to be the standard judgement of fetal aneuploidy and to define early pregnancy loss. However receiver operating characteristic curve shows 0.607 which is somewhat insufficient numerical value to expect the obvious result. To deduct the clinically practical cutoff value in spite of ambiguous supposition, larger sample group will be needed.
In conclusion, low maternal AMH level does appear to be a clinical age-independent marker of fetal aneuploidy in early pregnancy losses but FSH level was not predicting factor of expecting fetal aneuploidy in our study. In near future, further study with prospective manner will be needed to confirm the relationship between serum AMH level and aneuploidy risk in early pregnancy loss.
References
1. Boue J, Bou A, Lazar P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology 1975;12:11-26. PMID: 1162621.


2. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001;2:280-291. PMID: 11283700.


3. Kuliev A, Verlinsky Y. Meiotic and mitotic nondisjunction: lessons from preimplantation genetic diagnosis. Hum Reprod Update 2004;10:401-407. PMID: 15319376.


4. Pellestor F, Andreo B, Anahory T, Hamamah S. The occurrence of aneuploidy in human: lessons from the cytogenetic studies of human oocytes. Eur J Med Genet 2006;49:103-116. PMID: 16530707.


5. Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6,733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online 2003;6:54-59. PMID: 12626143.


6. Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril 1995;64:382-391. PMID: 7615118.


7. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimüllerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril 2004;82:1323-1329. PMID: 15533354.


8. Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve: should we perform tests of ovarian reserve routinely? Hum Reprod 2006;21:2729-2735. PMID: 16936296.


9. Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril 2010;93:855-864. PMID: 19046583.


10. Josso N, Picard JY, Rey R, di Clemente N. Testicular anti-Mullerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev 2006;3:347-358. PMID: 16816803.

11. Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril 2007;88:539-546. PMID: 17559842.


12. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 2002;77:357-362. PMID: 11821097.


13. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 2005;83:979-987. PMID: 15820810.


14. Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod 2003;18:328-332. PMID: 12571169.


15. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab 2006;91:4057-4063. PMID: 16804046.


16. La Marca A, Giulini S, Orvieto R, De Leo V, Volpe A. Anti-Mullerian hormone concentrations in maternal serum during pregnancy. Hum Reprod 2005;20:1569-1572. PMID: 15734752.


17. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2007;134:196-201. PMID: 17335955.


18. Harlap S, Shiono P, Pellegrin F, Golbus M, Bachman R, Mann J, et al. Chromosome abnormalities in oral contraceptive breakthrough pregnancies. Lancet 1979;1:1342-1343. PMID: 87796.

19. Kaufman MH. The teratogenic effects of alcohol following exposure during pregnancy, and its influence on the chromosome constitution of the pre-ovulatory egg. Alcohol Alcohol 1997;32:113-128. PMID: 9105505.


20. Narchi H, Kulaylat N. High incidence of Down's syndrome in infants of diabetic mothers. Arch Dis Child 1997;77:242-244. PMID: 9370905.



21. Torfs CP, van den Berg BJ, Oechsli FW, Christianson RE. Thyroid antibodies as a risk factor for Down syndrome and other trisomies. Am J Hum Genet 1990;47:727-734. PMID: 2145758.


22. Haadsma ML, Mooij TM, Groen H, Burger CW, Lambalk CB, Broekmans FJ, et al. A reduced size of the ovarian follicle pool is associated with an increased risk of a trisomic pregnancy in IVF-treated women. Hum Reprod 2010;25:552-558. PMID: 19920066.


23. Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB. Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril 1999;71:715-718. PMID: 10202884.


24. Plante BJ, Beamon C, Schmitt CL, Moldenhauer JS, Steiner AZ. Maternal antimullerian hormone levels do not predict fetal aneuploidy. J Assist Reprod Genet 2010;27:409-414. PMID: 20490648.



25. Setti AS, de Almeida Ferreira Braga DP, de Cassia Savio Figueira R, de Castro Azevedo M, Iaconelli A Jr, Borges E Jr. Are poor responders patients at higher risk for producing aneuploid embryos in vitro? J Assist Reprod Genet 2011;28:399-404. PMID: 21110079.


26. Thum MY, Abdalla HI, Taylor D. Relationship between women's age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril 2008;90:315-321. PMID: 17953958.


27. La Marca A, De Leo V, Giulini S, Orvieto R, Malmusi S, Giannella L, et al. Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Investig 2005;12:545-548.


28. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 2006;21:3103-3107. PMID: 16923748.


29. Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril 2008;90:395-400. PMID: 17919608.


30. Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod 2007;22:1837-1840. PMID: 17485437.


31. van Montfrans JM, Dorland M, Oosterhuis GJ, van Vugt JM, Rekers-Mombarg LT, Lambalk CB. Increased concentrations of follicle-stimulating hormone in mothers of children with Down's syndrome. Lancet 1999;353:1853-1854. PMID: 10359418.


32. van Montfrans JM, van Hooff MH, Huirne JA, Tanahatoe SJ, Sadrezadeh S, Martens F, et al. Basal FSH concentrations as a marker of ovarian ageing are not related to pregnancy outcome in a general population of women over 30 years. Hum Reprod 2004;19:430-434. PMID: 14747192.


33. Massie JA, Burney RO, Milki AA, Westphal LM, Lathi RB. Basal follicle-stimulating hormone as a predictor of fetal aneuploidy. Fertil Steril 2008;90:2351-2355. PMID: 18178189.


-
METRICS

-
- 13 Crossref
- 3,471 View
- 52 Download
- Related articles in Obstet Gynecol Sci