|
 |
- Search
| Obstet Gynecol Sci > Volume 59(4); 2016 > Article |
Abstract
Ovarian tumors are relatively rare in children and adolescent. The incidence of malignancies in these groups is 1% to 1.5%. The common histologic type is non-epithelial type such as germ cell tumors or sex cord-stromal tumors and only 10% to 17% of those are epithelial tumors. It is important to accurately diagnose in the early these rare tumors for proper staging and treatment to save the patient's life and fertility. We present a case of a 13-year-old girl with a giant ovarian mucinous borderline tumor.
Ovarian tumors are rare in children and adolescents. The incidence of malignancies in these groups is 1% to 1.5%, and the common histologic type is non-epithelial type such as germ cell tumors or sex cord-stromal tumors [1]. Epithelial ovarian cancers are commonly observed in adults, and the mean age at diagnosis of ovarian cancer is 63 years [2]. It is important to correctly diagnose these rare tumors in young women in order to assure proper treatment and to prevent mortality and preserve fertility. We present a case of a 13-year-old girl with a giant ovarian mucinous borderline tumor.
A 13-year-old girl who presented with painless abdominal distension over five months was referred to our institution for diagnosis and treatment in June 2015. She had no medical history with the exception of abdominal distension and amenorrhea. The last menstrual cycle was 10 months prior. Her menstrual cycle had been irregular since she experienced menarche at the age of 12 years. There was no reported use of oral contraceptives, and she was not known to be sexually active. Her physical examination showed abdominal distension and a firm mass without tenderness, extending from the pelvis to the umbilicus.
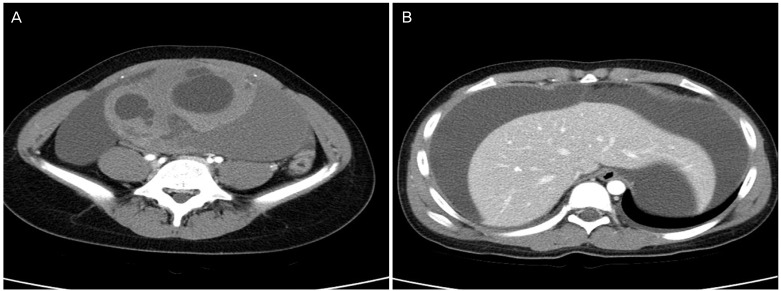
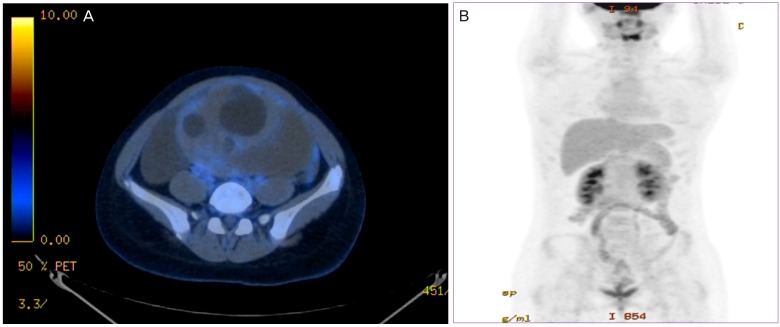
On tumor marker analysis, CA 19-9 (2,581 U/mL) and CA 125 (284 U/mL) were highly elevated, but carcinoembryonic antigen and ╬▒-fetoprotein were within normal ranges. Routine blood analyses showed normal renal and liver function with the exception of elevated alkaline phosphatase (172 IU/L). Computed tomography (CT) images of the abdomen and pelvis revealed an intra-abdominal cystic mass (17.5├Ś14├Ś9.2 cm) with hyper-attenuated solid portions, accompanied by large amounts of fluid collection in the entire abdomen and pelvic cavity (Fig. 1). An 18F-fluorodeoxyglucose positron emission tomography/CT scan was performed for possible detection of the malignant tumor and metastases. There was a large ovarian cystic mass with marked ascites in the perihepatic and perisplenic spaces, but no definite fluorodeoxyglucose uptake was seen either in large ovarian mass or other intaperitoneal and pelvic organs (Fig. 2).
Although CT scan could not identify the origin of the mass, a huge adnexal malignancy was strongly suspected, and an exploratory laparotomy was recommended. Laparotomy was performed with a midline incision. On surgical exploration, a large mass (19├Ś15├Ś8.5 cm, 1,584 g) was found to originate from the left ovary. The right adnexa was grossly normal. The left ovarian tumor had a smooth external surface but showed increased vascularity on the tumor surface. There was approximately 2,800 mL ascites in the abdomen, and cytologic analysis was performed. Because of the absence of normal ovarian tissue, a left salpingo-oophorectomy was performed. The surgical specimen was sent to pathology for frozen biopsy, and an ovarian mucinous borderline tumor was confirmed. Appendectomy and omentectomy were then performed. Examination of the pelvis, abdominal walls, and peritoneum was not indicative of implants or metastases.
The early postoperative course was uncomplicated, and CA 125 and CA 19-9 levels decreased to 175 and 180 U/mL, respectively, on the third postoperative day. The final pathologic result showed a left ovarian mucinous borderline tumor without metastasis, although tumor cells were found in the ascites fluid. The patient was treated successfully with complete resection, and her fertility was preserved. Serum CA 125 and CA 19-9 levels normalized three months after surgery. The patient did not undergo adjuvant treatment and continues to undergo close follow-up every three months, and showed no evidence of disease recurrence at 12 months from initial diagnosis.
Ovarian tumors in children are uncommon, with an incidence of neoplastic ovarian masses estimated at 2.6 cases per 100,000 girls each year [3]. Although epithelial ovarian cancer is common in the adult, it identifies only 1.9% of all ovarian neoplasm in the childhood [4]. The histologic subtypes of epithelial ovarian tumors in children are only serous and mucinous tumors, and are more commonly serous than mucinous [5]. In addition borderline epithelial ovarian tumors are about 70% of all epithelial neoplasm (21% of all ovarian malignancies) in women aged less than 25 years [6]. A pervious study was reported on 19 cases of epithelial ovarian neoplasm in children at their institution from 1988 to 2001 [7]. The mean age was 13.9┬▒4 years and four serous borderline tumors were diagnosed in this group. There was no mucinous borderline tumor.
Most of patients presented with early stage diseases. However, it is not easy to diagnose such tumors because symptoms are not typical. Preoperative diagnosis indicate suspicion of a malignancy in 80.0% of adolescent patients, based on imaging study and CA 125 level [8]. In contrast to germ cell tumors, epithelial ovarian tumors frequently may indicate an elevation of CA 125 level. Similarly, the large mass of this case showed the possibility of malignancy with highly elevated epithelial tumor markers (CA 19-9 and CA 125). Although the usual surgery for obviously benign ovarian tumors in children is performed via laparoscopy, laparoscopy is contraindicated in patients with suspicion of malignancy because of concerns regarding spillage of malignant tumor contents. The standard treatment for ovarian malignancy in them is conservative surgery followed by adjuvant chemotherapy, depending on stage and histology. According to the American College of Obstetricians and Gynecologists, the recommended surgical treatment for ovarian borderline malignancy includes resection of all visible disease, an omentectomy, and an appendectomy if a mucinous tumor is present and also suggest either a unilateral salpingo-oophorectomy or ovarian cystectomy for fertility sparing procedures [9].
Epithelial borderline tumors have an excellent prognosis. However, if the tumors relapse, the five-year survival rate is approximately 81%; if the tumors become malignant, the five-year survival rate is reduced to 68% [10]. In addition, the recurrence rate within first year after treatment in children and adolescents is about 75% and within the second year after initial treatment it is 90% [11]. Therefore, long and careful follow-up are critical to observe for disease recurrence by surveillance pelvic imaging and tumor markers (CA 19-9 and CA 125). In the adolescent, an early diagnosis for ovarian tumors is required to determine the direction of treatment. It is important to detect the possibility of malignancy in the early due to an effect on the future fertility and ovarian function. The goals of treatment for children and adolescents are to exterminate the disease, and restore the uterus and ovarian function for conservation of reproductive potential.
References
1. Wootton-Gorges SL, Thomas KB, Harned RK, Wu SR, Stein-Wexler R, Strain JD. Giant cystic abdominal masses in children. Pediatr Radiol 2005;35:1277-1288. PMID: 16151789.


2. Duska LR, Tew WP, Moore KN. Epithelial ovarian cancer in older women: defining the best management approach. Am Soc Clin Oncol Educ Book 2015;e311-e321. PMID: 25993191.


3. Skinner MA, Schlatter MG, Heifetz SA, Grosfeld JL. Ovarian neoplasms in children. Arch Surg 1993;128:849-853. PMID: 8343057.


4. Zhang M, Jiang W, Li G, Xu C. Ovarian masses in children and adolescents: an analysis of 521 clinical cases. J Pediatr Adolesc Gynecol 2014;27:e73-e77. PMID: 24157281.


5. Lack EE, Young RH, Scully RE. Pathology of ovarian neoplasms in childhood and adolescence. Pathol Annu 1992;27 Pt 2:281-356. PMID: 1316599.

6. You W, Dainty LA, Rose GS, Krivak T, McHale MT, Olsen CH, et al. Gynecologic malignancies in women aged less than 25 years. Obstet Gynecol 2005;105:1405-1409. PMID: 15932836.


7. Morowitz M, Huff D, von Allmen D. Epithelial ovarian tumors in children: a retrospective analysis. J Pediatr Surg 2003;38:331-335. PMID: 12632344.


8. Aggarwal A, Lucco KL, Lacy J, Kives S, Gerstle JT, Allen L. Ovarian epithelial tumors of low malignant potential: a case series of 5 adolescent patients. J Pediatr Surg 2009;44:2023-2027. PMID: 19853767.


9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007;110:201-214. PMID: 17601923.


10. Lazarou A, Fotopoulou C, Coumbos A, Sehouli J, Vasiljeva J, Braicu I, et al. Long-term follow-up of borderline ovarian tumors clinical outcome and prognostic factors. Anticancer Res 2014;34:6725-6730. PMID: 25368281.

11. Chaopotong P, Therasakvichya S, Leelapatanadit C, Jaishuen A, Kuljarusnont S. Ovarian cancer in children and adolescents: treatment and reproductive outcomes. Asian Pac J Cancer Prev 2015;16:4787-4790. PMID: 26107241.


-
METRICS

-
- 2 Crossref
- 2,849 View
- 34 Download
- Related articles in Obstet Gynecol Sci
-
A case of mixed germ cell tumor of the ovary.1991 December;34(12)
A report of two cases of malignant mixed Mullerian tunor of the ovary.1991 December;34(12)
A case of ovarian fibroma with teratoma of the ovary.1992 January;35(1)
Two cases of theca cell tumor of the ovary.1992 April;35(4)
One case of malignant mixed mesodermal(Mullerian) tumor of the ovary .1992 September;35(9)