 |
 |
- Search
| Obstet Gynecol Sci > Volume 59(6); 2016 > Article |
Abstract
Objective
Methods
Results
Supplemental Video
References
Table┬Ā1
Baseline characteristics (n=57)
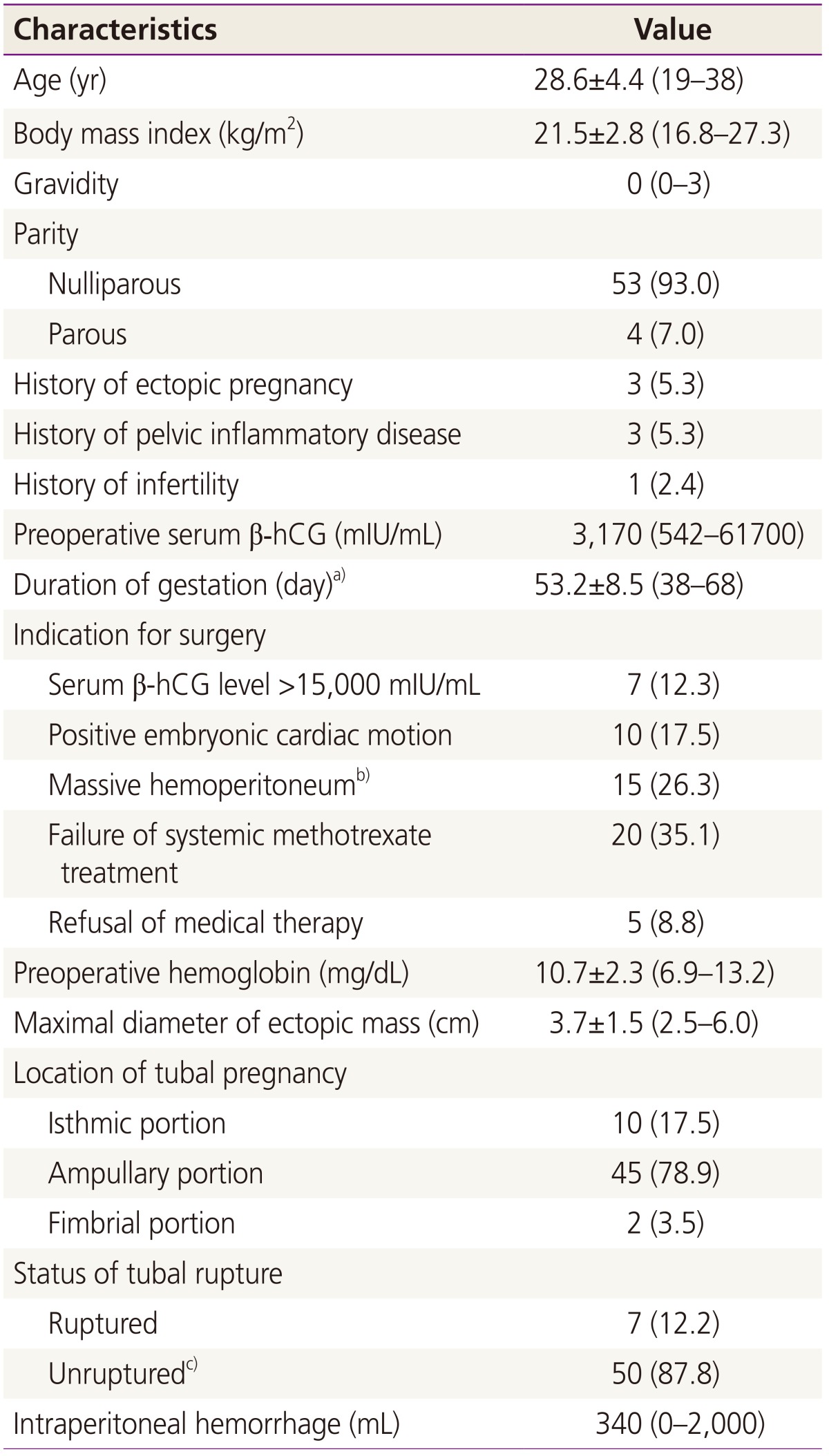
Data are represented as the mean┬▒standard deviation (range), median (range), or number (%).
╬▓-hCG, ╬▓-human chorionic gonadotropin.
a)Duration of gestation was calculated as the period from the date of the last menstrual period to the date of surgery; b)Massive hemoperitoneum was suspected on transvaginal sonography; c)Of 50 cases, 9 had a leaking tubal pregnancy, indicating an unruptured tubal pregnancy with active bleeding from the fimbrial orifice.
Table┬Ā2
Surgical results (n=57)

Data are represented as number (%) or mean┬▒standard deviation (range).
╬▓-hCG, ╬▓-human chorionic gonadotropin.
a)Defined as the completion of intended tube-preserving surgery without additional rescue therapy such as salpingectomy or methotrexate injection; b)Defined as the difference between the preoperative hemoglobin level and the level at postoperative day 1; c)Calculated as the period from the date of the surgery to the date of achieving a serum ╬▓-hCG of less than 5 mIU/mL; d)A tubal patency test using hysterosalpingography was performed in only 15 patients 3 months after achieving a serum ╬▓-hCG level of less than 5 mIU/mL.
- TOOLS
-
METRICS

-
- 5 Crossref
- 3,120 View
- 36 Download
- Related articles in Obstet Gynecol Sci
-
A clinical survey of ectopic pregnancy.1992 July;35(7)
A clinical survey of ectopic pregnancy.1993 June;36(6)
A Clinical Study Following Macrosurgical Procedures for Tubal REversal.1997 March;40(3)
A Case of Laparoscopic Conservative Surgery for Ovarian Pregnany.1998 August;41(8)




















