 |
 |
- Search
| Obstet Gynecol Sci > Volume 66(5); 2023 > Article |
|
Abstract
Objective
This study evaluated the association between pretreatment total lymphocyte count (TLC) and overall survival (OS) in patients with recurrent cervical cancer.
Methods
We retrospectively reviewed 290 patients with recurrent cervical cancer with definite complete responses to either definitive radiotherapy or concurrent chemoradiotherapy between January 2009 and December 2022. The associations between pretreatment TLC and progression-free survival (PFS) and OS rates were evaluated.
Results
Ninety-three patients (32%) had a pretreatment TLC <1,000 cells/mm3. Patients with a pretreatment TLC <1,000 cells/mm3 had lower treatment response rates than their counterparts (P=0.045). The OS and PFS rates were significantly higher in patients with pretreatment TLC ≥1,000 cells/mm3 than in those with pretreatment TLC <1,000 cells/mm3 (10.74 vs. 3.89 months, P<0.0001; 8.32 vs. 4.97 months, P=0.042; respectively). Moreover, pretreatment TLC ≥1,000 cells/mm3 was identified as an independent prognostic factor for OS in both univariate analysis (hazard ratio [HR], 0.57; 95% conficence interval [CI], 0.44-0.74; P<0.001) and multivariate analysis (HR, 0.64; 95% CI, 0.47-0.86; P=0.003). However, TLC ≥1,000 cells/mm3 was identified as a prognostic factor for PFS only in univariate analysis (HR, 0.71; 95% CI, 0.51-0.99; P=0.043) but not in the multivariate analysis (HR, 0.81; 95% CI, 0.55-1.18; P=0.3).
Cervical cancer is the second most common gynecological cancer worldwide [1]. Although there has been a downward trend in the mortality rate associated with cervical cancer, its incidence and mortality rates remain high in low- and middle-income countries. Global information has recently reported that the incidence and age-standardized mortality rates of cervical cancer in the Southeast Asian population are 17.8 and 10.0 per 100,000 people, respectively [1]. In Thailand, cervical cancer is the second most common gynecological malignancy after breast cancer [2].
Cervical cancer treatment is based on the disease stage. Concurrent chemoradiotherapy (CRT) is the standard mainstay treatment for early to locally advanced stages of the disease [3]. However, most recurrences develop within 3 years of completing radiotherapy [4,5]. Moreover, patients who develop recurrent diseases have a poor survival rate [6,7]. The treatment for recurrent disease also varies and depends on the site of recurrence [8,9]. Lymphocytes serve as markers of host antitumor immunity. The pretreatment total lymphocyte count (TLC) is associated with the prognosis of many cancers [10]. According to immunosurveillance theory, primary tumors can create a microenvironment and secrete various types of chemical substances. This can cause apoptosis or downregulation of lymphocytes, leading to lymphopenia, which serves as a host immune evasion mechanism for tumors [11,12].
Previous studies have demonstrated that pretreatment lymphopenia is associated with poor oncological outcomes in patients diagnosed with cervical cancer, especially in those with locally advanced-stage disease [13-15]. Pretreatment TLC has also been associated with survival outcomes in patients with recurrent cancers such as leukemia and head, neck, and esophageal cancers [16-18]. Although previous studies [13,14] have shown that pelvic radiation can also lead to lymphopenia status, it might affect the survival outcome. However, data regarding pretreatment TLC and survival outcomes in patients with recurrent cervical cancer are limited, especially for patients who have received definitive radiation-based therapy and might have had lymphopenia induced by previous CRT [8,19,20]. Furthermore, the results of existing studies on pretreatment TLC and survival outcomes for recurrent cervical cancer are contradictory and limited by small sample sizes.
Therefore, this study aimed to evaluate pretreatment TLC as a significant prognostic factor in a cohort of patients with recurrent cervical cancer who underwent definitive radiotherapy.
This study was conducted in accordance with the principles of the Declaration of Helsinki, as revised in 2013, and with the checklists and guidelines of strengthening the reporting of observational studies in epidemiology. All data were reviewed after approval was obtained from the Human Ethics Committee of the Faculty of Medicine (Prince of Songkla University REC 65-485-12-1). The requirement for informed consent was waived owing to the retrospective nature of the study.
The medical data of patients with recurrent cervical cancer who underwent prior definitive radiotherapy or concurrent CRT at Songklanagarind Hospital between January 2009 and December 2022 were retrospectively reviewed, with all patients kept anonymous during the entire study period. Patients with confirmed squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma based on tumor histology who had previously undergone complete definitive radiotherapy or CRT were included in this study. Patients who did not have any pretreatment TLC data (within 1 week before treatment) were excluded, as were those with active systemic infection, human immunodeficiency virus infection, hematologic or autoimmune disorders, other malignancies, or concurrent steroid use.
All eligible patients were primarily treated with radiotherapy or CRT and followed up in an outpatient clinic, as previously described [15]. Recurrence was defined as the appearance of symptoms and radiological or histological evidence of recurrence after a complete response to treatment. Detailed patient data, including demographic data, International Federation of Gynecology and Obstetrics (FIGO) stage, age at recurrence, sites of recurrent disease, treatment modalities used after the diagnosis of recurrence, and most recent laboratory data before the initiation of treatment, were obtained from medical records. A pretreatment TLC less than 1,000 cells/mm3 was defined as lymphopenia, in accordance with commonly accepted references [21].
The treatment modalities used after disease recurrence at our institution were as follows. Patients underwent local ablative therapy, such as surgery or radiation, if they had a single localized lesion. Systemic chemotherapy was administered if patients were not eligible for local therapy. Symptomatic palliative care was provided to patients who either had a poor functional status or refused chemotherapy. At our institute, all patients were scheduled for follow-up at an outpatient clinic at the end of treatment. Gynecological examinations were performed approximately every 3 months during the first 2 years, every 6 months during the 3 to 5 years, and then annually after 5 years. Chest radiography was performed annually. In addition, we performed imaging studies, such as computed tomography or magnetic resonance imaging, to define the disease response to therapy. Treatment responses were categorized according to the response evaluation criteria in solid tumors 1.1 [22]. Disease recurrence was defined as a reappearance of the disease after evidence of a definite complete response more than 6 months after CRT. Progression-free survival (PFS) was defined as the duration from the date of treatment initiation at the time of diagnosis of recurrence to the date of disease reappearance/progression or the date of the last follow-up. Overall survival (OS) was defined as the duration from the date of treatment initiation at the time of recurrence diagnosis to the date of the last follow-up or death.
The sample size was calculated from the survival analysis by The Lachin and Foulkes method 1986 (R package “GSDesign”; function nSurvival). The significance level (alpha) was set to 0.05, and the power of the test (1-β) was 0.80. The hazard ratios of patients with pretreatment TLC ≥1,000 cells/mm3 and those with pretreatment TLC <1,000 cells/mm3 from a previous study [15] were used for sample size calculation. Briefly, the hazard rates were 0.46 and 0.77; respectively. A dropout rate of 20% was considered. Ultimately, 278 patients were included in this study.
Data analysis was performed using R software version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria). Patient demographic data were analyzed using the Wilcoxon rank-sum test for continuous variables. Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test. PFS and OS were assessed using the Kaplan-Meier method and statistically compared using the log-rank test. Univariate and multivariate analyses were conducted using the log-rank test and Cox proportional hazards model, respectively. Statistical significance was set at P<0.05.
A total of 290 patients with recurrent cervical cancer who previously received complete definitive radiation-based therapy were included in this study. The demographic data of all the patients are summarized in Table 1. The median age of the entire cohort was 52 years (range, 45-60). Most patients were diagnosed with squamous cell carcinoma (67.2%), followed by adenocarcinoma (26.2%), and adenosquamous carcinoma (6.6%). Most patients (47.2%) initially had stage II disease. Recurrence occurred at extra-irradiated (43.1%), intra-irradiated (28.3%), or both extra-irradiated and intra-irradiated sites (28.6%). Most patients underwent systemic chemotherapy (81.0%), followed by symptomatic treatment (10.0%), localized radiation (69.0%), or a combination of these treatments (2.1%). Platinum-based chemotherapy was administered to most patients (69.0%), either as a single agent or in combination with other chemotherapeutic regimens (13.1%, 55.9%; respectively).
In this study, 93 patients (32%) were diagnosed with pretreatment lymphopenia. Demographic data were compared between patients with TLC <1,000 cells/mm3 and those with TLC ≥1,000 cells/mm3, as shown in Table 1. Factors such as median age, tumor histology, initial FIGO stage of the disease, and site of recurrent disease were not significantly different between the two groups. However, more patients without lymphopenia were administered combined platinum-based chemotherapy than those with lymphopenia (P=0.004).
The treatment outcomes are presented in Table 2. A total of 109 patients (42.7%) responded to the treatment regimens; of these, 25 (9.8%) demonstrated a complete response. Nevertheless, 68 patients (26.7%) did not respond to treatment, while 78 (30.6%) exhibited stable disease during treatment.
Patients with pretreatment lymphopenia had lower treatment response rates than their counterparts (P=0.045; Table 2). Furthermore, we performed pairwise comparisons and found that patients with TLC ≥1,000 cells/mm3 had a significantly greater disease response than those with TLC <1,000 cells/mm3 (complete response: progressive disease and partial response: progressive disease, 11.6% vs. 5.4%, P=0.028; 35.4% vs. 27.1%, P=0.024; respectively). We have also shown the treatment outcomes according to treatment modality in Supplementary Table 1.
The median OS for the entire cohort was 8 months (95% confidence interval [CI], 7.1-9.6 months). A comparison of OS between the two groups stratified by pretreatment TLC levels is shown in Fig. 1. Patients with pretreatment TLC ≥1,000 cells/mm3 had significantly higher OS than those with TLC <1,000 cells/mm3 (10.74 vs. 3.89 months, P<0.0001; Fig. 1A).
Similarly, patients with pretreatment TLC ≥1,000 cells/mm3 had significantly higher PFS than those with TLC <1,000 cells/mm3 (8.32 vs. 4.97 months, P=0.042; Fig. 1B). Time-varying PFS stratified by time at <24 and ≥24 months was analyzed, and it was observed that patients with a pretreatment TLC ≥1,000 cells/mm3 had a higher PFS than their counterparts in the first 24 months (P=0.028). However, PFS was not significantly different between the two groups after 24 months (P=0.994).
Univariate and multivariate analyses of PFS are shown in Table 3. Symptom-only treatment or disease progression during treatment was a significantly poor predictor of PFS in both the univariate and multivariate analyses. Pretreatment TLC ≥1,000 cells/mm3 was a good predictor of PFS in the univariate analysis (hazard ratio [HR], 0.71; 95% CI, 0.51-0.99; P=0.043). However, pretreatment TLC ≥1,000 cells/mm3 was not an independent prognostic factor for PFS in the multivariate analysis (HR, 0.81; 95% CI, 0.55-1.18; P=0.3).
Table 4 presents the univariate and multivariate analyses of OS. The combination of platinum-based chemotherapy and symptomatic treatment was a significant prognostic factor only in univariate analysis. Progressive or stable disease during treatment was an independent poor prognostic factor for OS. Moreover, the results demonstrated that pretreatment TLC ≥1,000 cells/mm3 was a significant independent prognostic factor for OS in both univariate (HR, 0.57; 95% CI, 0.44-0.74; P<0.001) and multivariate analyses (HR, 0.64; 95% CI, 0.47-0.86; P=0.003).
The findings of the current study indicate that patients with pretreatment TLC ≥1,000 cells/mm3 achieved a better treatment response rate and had higher PFS and OS rates than those with TLC <1,000 cells/mm3. The time-varying effect of the pretreatment TLC was evaluated. The association between pretreatment TLC ≥1,000 cells/mm3 and PFS diminished after >24 months. Our multivariate analysis revealed that palliative symptomatic treatment was a poor independent prognostic factor for PFS but not OS. Furthermore, we identified that pretreatment TLC ≥1,000 cells/mm3 was an independent prognostic factor for OS in patients with recurrent cervical cancer.
Previous studies have reported the association of pretreatment TLC with the prognosis of various types of cancer, including cervical cancer, especially locally advanced-stage disease [13-15]. However, data on the importance of pre-treatment TLC in predicting the prognosis of recurrent cervical cancer are not well established. Ida et al. [19] observed that the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, and prognostic nutritional index (PNI) were significantly associated with OS in univariate analyses of recurrent cervical cancer. Moreover, a PNI <46.70 was an independent prognostic factor for OS (HR, 3.767; 95% CI, 1.750-8.109; P=0.001) in patients who developed recurrent cervical cancer after CRT. Ittiamornlert and Ruengkhachorn [20] reported that NLR ≥3.6 was an independent prognostic factor for PFS and OS in recurrent and progressive cervical cancer. However, pre-treatment TLC was not associated with PFS or OS in that study. Recently, Taguchi et al. [8] also reported that pretreatment TLC at the time of relapse was not associated with survival outcomes in patients with recurrent cervical cancer (HR, 0.99; 95% CI, 0.9985-1.0000; P=0.08). However, the authors did not describe the cutoff points for TLC levels. The differences between the findings of these studies and ours could be attributed to differences in patient characteristics, sample sizes, and heterogeneity in the cutoff points of the pretreatment TLC levels used in these studies.
The exact mechanisms by which lymphocyte count affects survival remain elusive. However, peripheral lymphocytes secrete various chemokines and cytokines that prevent cancer progression and metastasis. This antitumor function is impaired by chemical substances created and secreted into the tumor microenvironment [11,12]. Moreover, lymphopenia is associated with survival in patients diagnosed with various types of cancer, including cervical cancer [10].
Immunotherapy serves as an immunomodulator, and its mechanism of action is dependent on cytotoxic T-cell lymphocytes [23]. According to the latest National Comprehensive Cancer Network guidelines, immunotherapy has emerged as the first-line treatment for patients with recurrent cervical cancer whose tumors express programmed death-ligand 1 [9]. Recently, lymphopenia has been associated with lower response rates and poor survival outcomes in various types of cancer treated with immunotherapy [24,25]. In addition, pre-treatment TLC may serve as a significant biomarker for triaging patients who may benefit the most from immunotherapy.
The limitations of this study include its retrospective nature and the fact that some patients may have been lost to follow-up, along with some missing data. Furthermore, the functional status of patients could not be ascertained from their medical charts. Second, this was a single-center study. Moreover, the treatment regimens were not similarly balanced in both groups, as more patients with pretreatment TLC ≥1,000 cells/mm3 were treated with combined systemic platinum-based chemotherapy than their counterparts. Moreover, the tumor load at the time of recurrence was not specified.
Despite these limitations, this study included a large number of patients with recurrent cervical cancer and showed that pre-treatment TLC was associated with time-varying PFS for the first 24 months. Moreover, the results of this study provide further information that may support the use of pretreatment TLC as a significant independent prognostic factor associated with survival in patients diagnosed with recurrent cervical cancer.
To the best of our knowledge, our study is the first to highlight a strong association between pretreatment TLC and survival in patients with recurrent cervical cancer. Hence, pretreatment TLC can be used as an important factor in predicting tumor response and patient survival. Furthermore, this laboratory investigation is less expensive and is available in most hospitals. With the emergence of immunotherapy, we hypothesized that pretreatment TLC could be used as a biomarker to triage patients with recurrent cervical cancer who might benefit the most from immunotherapy. However, more data are required and further prospective studies should be performed to confirm this application.
In conclusion, pretreatment TLC is associated with treatment response in recurrent cervical cancer and is an independent prognostic factor associated with the survival outcome of patients with recurrent cervical cancer.
Acknowledgments
The authors thank the Department of Obstetrics and Gynecology, (blinded information) for providing us with the opportunity to perform this study.
Notes
Fig. 1
Overall survival (A) and progression-free survival (B) in patients with recurrent cervical cancer, stratified according to pretreatment total lymphocyte count. TLC, total lymphocyte count.
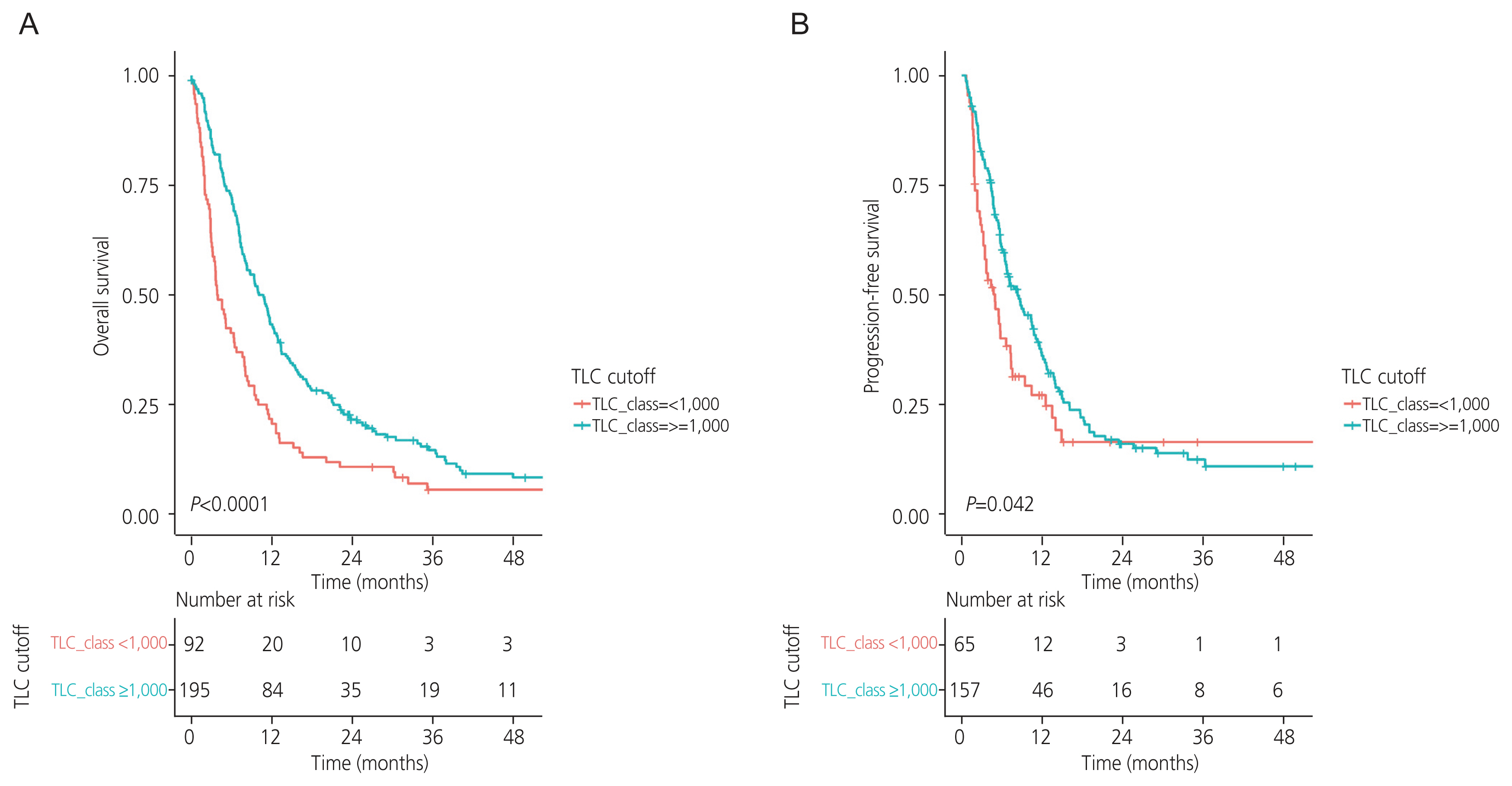
Table 1
Demographic data of patients stratified according to pretreatment total lymphocyte count
| Characteristic | Overall (n=290) | TLC <1,000 cells/mm3 (n=93) | TLC ≥1,000 cells/mm3 (n=197) | P-valuea) |
|---|---|---|---|---|
| Age | 52 (45.0 to 60.0) | 52 (45.0 to 61.0) | 52 (45.0 to 59.0) | 0.8 |
| Histology | 0.9 | |||
| AD | 76 (26.2) | 24 (25.8) | 52 (26.4) | |
| ASC | 19 (6.6) | 7 (7.5) | 12 (6.1) | |
| SCC | 195 (67.2) | 62 (66.7) | 133 (67.5) | |
| Site of recurrence | 0.3 | |||
| Extra-irradiated area | 125 (43.1) | 35 (37.6) | 90 (45.7) | |
| Intra-irradiated area | 82 (28.3) | 26 (28.0) | 56 (28.4) | |
| Intra- and extra-irradiated areas | 83 (28.6) | 32 (34.4) | 51 (25.9) | |
| Initial FIGO stage | 0.4 | |||
| I | 8 (2.8) | 3 (3.2) | 5 (2.5) | |
| II | 137 (47.2) | 38 (40.9) | 99 (50.3) | |
| III | 136 (46.9) | 48 (51.6) | 88 (44.7) | |
| IV | 9 (3.1) | 4 (4.3) | 5 (2.5) | |
| Treatment | <0.001 | |||
| CMT | 235 (81.0) | 65 (69.9) | 170 (86.3) | |
| CMT with local RT | 6 (2.1) | 1 (1.1) | 5 (2.5) | |
| RT | 20 (6.9) | 7 (7.5) | 13 (6.6) | |
| Symptomatic | 29 (10.0) | 20 (21.5) | 9 (4.6) | |
| Chemotherapy regimen | 0.004 | |||
| Single platinum | 38 (13.1) | 10 (10.8) | 28 (14.2) | |
| Combined platinum | 162 (55.9) | 42 (45.2) | 120 (60.9) | |
| Others | 90 (31.0) | 41 (44.0) | 49 (24.9) |
Table 2
Treatment outcomes of patients according to pretreatment lymphocyte count
| Characteristic | Overall (n=255) | TLC <1,000 cells/mm3 (n=74) | TLC ≥1,000 cells/mm3 (n=181) | P-valuea) |
|---|---|---|---|---|
| Response status | 0.045 | |||
| Complete response | 25 (9.8) | 4 (5.4) | 21 (11.6) | |
| Partial response | 84 (32.9) | 20 (27.1) | 64 (35.4) | |
| Progressive disease | 68 (26.7) | 28 (37.8) | 40 (22.1) | |
| Stable disease | 78 (30.6) | 22 (29.7) | 56 (30.9) |
Table 3
Univariate and multivariate analyses for factors associated with progression-free survival
Table 4
Univariate and multivariate analyses for factors associated with overall survival
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49.



2. ThaiCancerBase National Cancer Institute. Hospital-based cancer registry 2020 [Internet] Bangkok (TH): ThaiCancerBase National Cancer Institute; c2020 [cited 2022 Dec 26]. Available from: https://www.nci.go.th/e_book/hosbased_2563/index.html
.
3. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet 2021;155(Suppl 1):28-44.




4. Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1992;24:197-204.


5. Eifel PJ, Jhingran A, Brown J, Levenback C, Thames H. Time course and outcome of central recurrence after radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:1106-11.


6. Poolkerd S, Leelahakorn S, Manusirivithaya S, Tangjitgamol S, Thavaramara T, Sukwattana P, et al. Survival rate of recurrent cervical cancer patients. J Med Assoc Thai 2006;89:275-82.

7. Thanagumtorn K. Survival rate of recurrent cervical carcinoma. J Med Assoc Thai 2012;95(Suppl 3):S125-30.
8. Taguchi A, Nakajima Y, Furusawa A, Yoshino Y, Takao M, Kashiyama T, et al. High neutrophil-to-lymphocyte ratio is a predictor of short-term survival for patients with recurrent cervical cancer after radiation-based therapy. J Obstet Gynaecol Res 2021;47:1862-70.



9. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cervical cancer version 1, 2023 [Internet] Plymouth Meeting (PA): National Comprehensive Cancer Network; c2023 [cited 2023 Mar 15]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426
.
10. Rochet NM, Markovic SN, Porrata LF. The role of complete blood cell count in prognosis-watch this space! Oncol Hematol Rev 2012;8:76-82.

11. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol 2017;14:155-67.



12. Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol 2018;30:445-54.



13. Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016;140:76-82.



14. Shi F, Yoder AK, Mach C, Dalwadi S, Anderson ML, Hall TR, et al. Impact of hematologic toxicities during concurrent chemoradiation for cervical cancer. Obstet Gynecol Sci 2022;65:176-87.




15. Thiangphak E, Pruegsanusak K, Buhachat R. Pretreatment lymphocyte count as independent prognostic factor in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Int J Gynaecol Obstet 2022;159:672-8.



16. Inoue H, Shiozaki A, Fujiwara H, Konishi H, Kiuchi J, Ohashi T, et al. Absolute lymphocyte count and C-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncol Lett 2022;24:257.



17. Zhang Y, Dai K, Zhang Q, Huang Y, Feng Y, Bhardwaj D, et al. Normal absolute monocyte count in combination with normal/high absolute lymphocyte count at the time of relapse is associated with improved survival in patients with early relapsed acute myeloid leukemia. Cancer Invest 2021;39:550-8.


18. Park JC, Durbeck J, Clark JR. Predictive value of peripheral lymphocyte counts for immune checkpoint inhibitor efficacy in advanced head and neck squamous cell carcinoma. Mol Clin Oncol 2020;13:87.



19. Ida N, Nakamura K, Saijo M, Kusumoto T, Masuyama H. Prognostic nutritional index as a predictor of survival in patients with recurrent cervical cancer. Mol Clin Oncol 2018;8:257-63.


20. Ittiamornlert P, Ruengkhachorn I. Neutrophil-lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. BMC Cancer 2019;19:51.




21. Vasu S, Caligiuri MA. Lymphocytosis and lymphocytopenia. In: Kaushansky K, Lichtman MA, Prchal JT, Levi M, Press OW, Burns LJ, , editors. Williams hematology. 9th ed. New York (NY): McGraw-Hill Education; 2015. p. 1204.
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.


23. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64.




- TOOLS





















