Hydroxychloroquine in obstetrics: potential implications of the prophylactic use of hydroxychloroquine for placental insufficiency during pregnancy
Article information
Abstract
Proper placentation during early pregnancy is a key factor for maintaining a healthy pregnancy. Placental insufficiency leads to critical complications such as preeclampsia, fetal growth restriction, and fetal demise. These complications are often associated with pathological findings of restricted remodeling and obstructive lesions of the myometrial spiral arteries, which have high recurrence rates during subsequent pregnancies. Currently, there are no pharmacological interventions other than aspirin for the prevention of preeclampsia. Hydroxychloroquine (HCQ), a well-known antimalarial drug, reduces inflammatory and thrombotic changes in vessels. For decades, the use of HCQ for autoimmune diseases has resulted in the successful prevention of both arterial and venous thrombotic events and has been extended to the treatment of lupus and antiphospholipid antibody syndrome during pregnancy. HCQ reduces the risk of preeclampsia with lupus by up to 90%. Several recent studies have investigated whether HCQ improves pregnancy outcomes in women with a history of poor outcomes. In addition, in vitro and animal studies have demonstrated the beneficial effects of HCQ in improving endothelial dysfunction and alleviating hypertension and proteinuria. Therefore, we hypothesized that HCQ has the potential to attenuate the vascular inflammatory and thrombogenic pathways associated with placental insufficiency and conducted a multicenter clinical trial on the efficacy of combining aspirin with HCQ for pregnancies at high risk for preeclampsia in Korea. This study summarizes the potential effects of HCQ on pregnancies with placental insufficiency and the implications of HCQ treatment in the field of obstetrics.
Introduction
Hydroxychloroquine (HCQ) was first synthesized in 1950 and approved by the Food and Drug Administration for medical use as an antimalarial drug [1]. For decades, HCQ has been used as an antimalarial and disease-modifying antirheumatic drug [1,2]. When administered to treat systemic lupus erythematosus (SLE), HCQ has shown multiple therapeutic effects, including reduced thrombotic events, dyslipidemia, renal failure, and disease activity, as well as improved survival rates [3]. Recently, HCQ has emerged as a new agent in the field of obstetrics for the prevention of placenta-related disorders. The use of HCQ for SLE and other rheumatic diseases during pregnancy improves not only disease flares but also pregnancy-related complications, such as preeclampsia (PE), fetal growth restriction (FGR), and preterm delivery [4–6]. In the United Kingdom, rheumatologists and gynecologists have changed their practices and continue to administer HCQ when patients with autoimmune diseases become pregnant, with this trend increasing from 3% to 13% between 2001 and 2015 [7]. The 2020 American College of Rheumatology guidelines recommend HCQ administration for pregnant patients with SLE or antiphospholipid antibodies [2]. Therefore, feto-maternal experts have focused on the effects of HCQ on pregnancies at risk of PE, FGR, and fetal death in utero (FDIU) [8]. A growing body of evidence suggests that HCQ may attenuate vascular inflammatory and thrombogenic pathways commonly associated with placental insufficiency, such as PE and FGR. Several ongoing trials on HCQ for the prevention of PE or recurrent miscarriages are underway in France (NCT05237843), Denmark (NCT03305263), China (NCT05651373, NCT04918524, and NCT04569890), and Egypt (NCT04228263). In Korea, we have begun a multicenter clinical trial on the efficacy of combining aspirin with HCQ for pregnancies at a high risk of PE. In this review, we discuss the potential effects of HCQ on pregnancy, particularly among women with placental insufficiency.
Great obstetric syndromes
Placental insufficiency is a major complication associated with PE, FGR, FDIU, and preterm birth. Although the relationship between these conditions has not been established, most pregnancy complications are associated with placental dysfunction and they commonly overlap. Brosens et al. [9] referred to these as “great obstetric syndromes” and focused on the key role of placentation. Different degrees of restricted remodeling and obstructive lesions of the myometrial spiral arteries may be associated with pregnancy complications including PE, FGR, preterm labor, preterm premature rupture of membranes, spontaneous abortion, abruptio placentae, and placental infarction. The lack of spiral arteries has been observed in the central part of the placenta in severe PE. Additionally, vascular occlusion caused by a thrombus or acute atherosis in the spiral artery results in a more severe degree of placental insufficiency. These findings of vascular obstruction are also observed in the placentas of pregnant women affected by FGR [10,11]. Placentas associated with FGR undergo physiological changes limited to the decidual artery, resulting in a reduced spiral artery lumen compared with that in normal pregnancies [12]. The reduced lumen of the spiral artery may result in increased vascular resistance and decreased uterine blood flow to the fetus [13]. Fewer endovascular trophoblasts and less extensive fibrinoid changes in the wall of spiral arteries have been observed in late fetal death, defined as fetal death after 14 weeks of gestation [14]. In contrast, the proportion of endovascular trophoblasts in the decidual segment of the spiral arteries increases. These findings suggest that endovascular trophoblasts may be arrested in the decidua and interrupt the development of the myometrial spiral artery. Vascular endothelial cells are damaged by proinflammatory cytokines, reactive oxygen species, and anti-angiogenic factors (activin A, soluble fms-like tyrosine kinase-1 [sFlt-1], and soluble endoglin) that occur in PE, thus impairing the systemic reaction of the mother. The increase in vasoconstrictive substances (endothelin 1 and thromboxane A2), activation of the renin-angiotensin system, and decrease in nitric oxide and adhesion molecules that cause monocyte binding to vascular endothelial cells results in decreased maternal vascular endothelial cell function [15]. Therefore, the prevention and treatment of placental insufficiency should focus on the suppression of endothelial cell damage through anti-inflammatory and antioxidant treatments.
Recurrence of placenta insufficiency
Notably, there is a significant clinical association between PE, FGR, FDIU and preterm birth, such that a history of placental disease increases the risk of placenta-related disease in subsequent pregnancies. Therefore, the high recurrence rate of placental insufficiency places a significant burden on women planning subsequent pregnancies. A history of PE is considered the most important risk factor and predictor of PE development during subsequent pregnancies [16]. The recurrence rate varies depending on clinical factors; however, approximately 15–50% of women with a history of placental insufficiency may experience recurrence [17,18]. For example, several guidelines include a history of PE as an indication for aspirin use, and a previous systemic review that analyzed 15 clinical guidelines included PE as a “major” risk factor that is strongly associated with PE [19].
Approximately 15–20% of women with pregnancies involving PE have a history of pregnancies involving FGR. Furthermore, PE requiring early delivery is more likely to be associated with FGR than less severe PE in women who deliver at term and are at risk of recurrence. In the World Health Organization Antenatal Care Trial, 41,751 pregnancies were analyzed, and the risk of gestational hypertension was found to be higher (3.8% vs. 3.1%; odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1–1.7) for women with a history of FGR than for those with normal pregnancies [20].
Regarding FDIU, a recent meta-analysis showed that women who had experienced FDIU during a previous pregnancy had a higher pooled relative risk (RR) of PE (OR, 2.4; 95% CI, 1.7–3.4) than women with a normal pregnancy history [21]. Compared with the control group, women who experienced stillbirth were almost five times more likely to experience stillbirth recurrence during a subsequent pregnancy (OR, 4.77; 95% CI, 3.70–6.15) [22].
Preventive agents for placenta-related diseases
Low-dose aspirin is the only proven preventive medication used to reduce the risk of PE, a placenta-related disease. Several guidelines, including those of the American College of Obstetricians and Gynecologists [15], National Institute for Health and Care Excellence (United Kingdom), Society of Obstetricians and Gynaecologists of Canada [23], European Society of Cardiology [24], Society of Obstetric Medicine of Australia and New Zealand [25], and International Society for the Study of Hypertension in Pregnancy [26], have recommended the use of aspirin as a preventive agent for pregnancies at risk for PE. Although there are differences among clinical guidelines, most have specified the indications for aspirin use based on the maternal clinical risk factors. Of note, the American College of Obstetricians and Gynecologists guidelines include a history of pregnancy as a risk factor and an indication for aspirin use, highlighting the importance of recurrent placenta-related diseases. Aspirin use during high-risk pregnancies reduces PE by 10–50% [27–29] and FGR by 25–50% [30,31].
Several clinical trials have been conducted under the assumption that the anticoagulant effect of low-molecular-weight heparin can prevent PE and FGR; however, no trial has demonstrated a significant beneficial effect. In 2009, a pilot randomized controlled trial evaluated the effect of dalteparin in preventing the recurrence of PE, FGR, and placental abruption during pregnancy in women with a history of these complications [32]. Use of delteparin in high risk pregnancy of placenta insufficiency showed reduced rate of the primary outcome (5.5% [n=3/55] vs. 23.6% [n=13/55]; adjusted OR, 0.15; 95% CI, 0.03–0.70) [32]. In 2017, a randomized trial investigated the preventive effects of enoxaparin on PE and FGR among women with pregnancies considered to be at high risk based on their pregnancy history and found no difference in the rates of PE and FGR when enoxaparin with aspirin or aspirin alone was administered [33].
The absence of an alternative prophylactic medication, other than aspirin, to improve pregnancy outcomes for pregnant women at high risk of placenta-related disease highlights the need to investigate new agents for disease prevention.
Potential mechanism of action of HCQ on pregnancies at risk for placenta-related diseases
Several mechanisms have been proposed for the therapeutic effects of HCQ. First, the inhibition of lysosomal activity and Toll-like receptor signaling results in immunomodulatory and anti-inflammatory effects [1]. Lysosomes are primarily involved in antigen presentation, a critical step in the adaptive immune response. Autophagy is involved in antigen presentation and immune stimulation [34]. HCQ may interfere with the activities of lysosomes and autophagosomes and, consequently, immune activation [35,36]. HCQ may also inhibit Toll-like receptor signaling by altering the pH of endosomes and the expression of proinflammatory cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor [37]. PE induces an exaggerated immune response against trophoblasts, leading to placental oxidative stress and ischemia [38]. This maternal-fetal immunological conflict may respond to the immunomodulatory effects of HCQ. Second, HCQ may have antithrombotic effects in autoimmune diseases with secondary coagulopathy caused by endothelial inflammation [39–41]. HCQ exerts protective endothelial effects in SLE, thereby reducing the incidence of thrombosis by 40–80% [42,43]. In an in vivo study using a mouse model of antiphospholipid syndrome, the antiphospholipid antibody-induced prothrombotic state and endothelial dysfunction were reversed by HCQ [30]. A study of the extension of the endothelial protective effect on PE that used an in vitro model of tumor necrosis factor-α and PE serum-induced dysfunction suggested that HCQ significantly attenuated the production of tumor necrosis factor-α and PE serum-induced endothelin-1 [44]. Third, oxidative stress and other types of stress cause syncytial trophoblast dysfunction and result in the overproduction of antiangiogenic factors such as sFlt-1 during syncytial trophoblast dysfunction [45]. HCQ alleviates syncytial trophoblast differentiation in cultured human trophoblasts and lowers the level of beta-human chorionic gonadotropin, a trophoblast differentiation marker, via the JAK signaling pathway [46]. Although the exact molecular mechanisms underlying the protective endothelial effects remain largely unclear, vascular endothelial injury and dysfunction associated with PE, resulting from the actions of antiangiogenic and proinflammatory leukocyte mediators, could be attenuated by the antithrombotic effects of HCQ [47].
In an in vitro experiment using BeWo and primary trophoblastic cells, HCQ restored trophoblast fusion affected by antiphospholipid antibodies [48]. According to a mouse model of obstetric antiphospholipid syndrome, HCQ prevented complement activation both in vivo and in vitro, placental insufficiency, and abnormal fetal brain development [49]. A recent study involving human umbilical vein endothelial cells reported that HCQ attenuates PE serum-induced endothelin-1 levels. Our recent study showed that HCQ alleviated hypertension and proteinuria and normalized soluble fms-like kinase-1 and endothelin-1 levels in the Nω-nitro-L-arginine methyl ester-induced PE rat model [50].
HCQ use and its effect on complications during pregnancy with autoimmune diseases
Lupus is most prevalent autoimmune disease during the reproductive years (20–30 years of age), and the incidence of pregnancy complications such as PE, FGR, FDIU, preterm delivery, and spontaneous abortion in women with lupus is higher than that in normal pregnancies [6,51–54]. As uncontrolled systemic inflammatory diseases worsen both pregnancy outcomes and underlying diseases, rheumatologists recommend maintaining medications during pregnancy [2]. Over the past few decades, drugs such as aspirin, prednisolone, and heparin have been administered during pregnancy to regulate maternal diseases and reduce fetal complications. Recently, the use of HCQ during pregnancy has been recommended for pregnant women with lupus or antiphospholipid antibody syndrome, based on reports that HCQ use may improve pregnancy outcomes and reduce disease exacerbations. In 2016, a meta-analysis including eight studies (comprising a total of 740 infants born to mothers with autoimmune diseases who were administered HCQ) found no significant decrease in the rates of stillbirth (OR, 0.69; 95% CI, 0.35–1.34), low birth weight (OR, 0.69; 95% CI, 0.21–2.27), or prematurity (OR, 1.75; 95% CI, 0.95–3.24) [55]. However, in 2021, a meta-analysis of nine studies including a total of 1,132 women with SLE reported that the use of HCQ during pregnancy effectively reduced gestational hypertension (OR, 0.41; 95% CI, 0.19–0.89), PE (OR, 0.35; 95% CI, 0.21–0.59), and preterm birth (OR, 0.55; 95% CI, 0.36–0.86) [6]. Although no comprehensive study has clarified the pathophysiological parallels between autoimmune diseases and placental insufficiency disorders, it can be assumed that autoimmune diseases have features similar to those of placental insufficiency disorders and are particularly characterized by vascular inflammation and thrombotic events. Therefore, HCQ, which can improve pregnancy outcomes in women with autoimmune diseases, may prevent placental insufficiency.
Safety of HCQ
No studies have been conducted on the safety of HCQ during pregnancy in women without autoimmune diseases. Controversy regarding the relationship between fetal safety and the use of HCQ to improve pregnancy outcomes in women without rheumatic diseases needs to be conducted through further research.
Meta-analyses of rheumatology studies showed that HCQ use during pregnancy is safe for the fetus. During a meta-analysis by Kaplan et al. [55] of 1,820 pregnancies of women with lupus, there were no differences in the risks of major fetal abnormalities when compared with the untreated group (OR, 1.13; 95% CI, 0.59–2.17). A meta-analysis by Duan et al. [6] that comprised 1,132 pregnancies of women with lupus showed that there was no discernible variance in the prevalence of major fetal abnormalities within the treated group compared with the untreated group (OR, 0.53; 95% CI, 0.14–2.04). In a nationwide cohort study by Andersson et al. [56] in Denmark (1,240,875 pregnancies), the fetal safety of 1,487 pregnancies exposed to 4-aminoquinolines (1,184 pregnancies exposed to chloroquine and 303 pregnancies exposed to HCQ) was compared with that of the unexposed group, and the prevalence of major fetal malformations was evaluated (prevalence: OR, 0.94; 95% CI, 0.59–1.52). Additionally, in subgroup analyses of HCQ exposure at approximately 4 weeks to 10 weeks of gestation (prevalence: OR, 0.78; 95% CI, 0.42–1.46) and HCQ exposure (prevalence: OR, 1.23; 95% CI, 0.50–3.04), no differences in the occurrence of major fetal malformations was observed [56].
Previous studies have evaluated the safety of HCQ during pregnancy and its association with fetal malformations. The most important factors related to effects on the fetus are the total exposure period, time of exposure, and dose of HCQ during pregnancy. The most vulnerable period for fetal malformations caused by drug exposure ranges from embryo implantation to 10 weeks of gestation, based on the start of the last menstrual period. A recent population-based cohort study by Huybrechts et al. [57] investigated the risk of major fetal malformations associated with exposure to HCQ during the first trimester and suggested a small increase in the risk of overall malformations associated with first-trimester HCQ use (unadjusted RR, 1.51; 95% CI, 1.27–1.81). The adjusted risk of major congenital anomalies was 33% higher for neonates with prenatal exposure of >400 mg/day during the first trimester (RR, 1.33; 95% CI, 1.08–1.65). However, the risk of fetal malformations does not increase with exposure of <400 mg/day during the first trimester (RR, 0.95; 95% CI, 0.60–1.50). No difference was observed in fetal malformations based on the exposure period (>60 or <60 days) during the first trimester.
Long-term HCQ treatment is associated with a risk of retinopathy caused by impaired metabolism of the retinal pigment epithelium and subsequent photoreceptor degeneration with autoimmune diseases during long-term use [58]. A study of 2,361 patients in the United States who had used HCQ continuously for at least 5 years suggested the potential risk of retinopathy, which reached 7.5%; furthermore, this risk varied with daily dosage (>5.0 mg/kg: OR, 5.67; 95% CI, 4.14–7.79) and exposure period (>10 years: OR, 3.22; 95% CI, 2.20–4.70) [59]. A study of 1,597 patients in Denmark who used HCQ showed a lower prevalence (1.6%) than that previously reported in a study performed in the United States [60]. In 2020, four major societies, the American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and American Academy of Ophthalmology-discussed HCQ treatment and created a consensus on its use in minimizing the risk of retinal toxicity [61]. Based on evidence that the incidence of retinal toxicity is 2% when less than 5 mg/kg per day is used for more than 10 years, these societies announced that surveillance for retinal toxicity is not required for those who have been using HCQ for less than 5 years.
The benefits and risks of HCQ use during pregnancy should be properly evaluated and decisions regarding its use should be based on the exposure period, dose, and gestational age.
In addition, there are no reports of antibiotic resistance in patients for whom HCQ has been used as an antimalarial drug. People should take a weekly dose of HCQ for at least 1 week before traveling to an area where malaria transmission occurs. It is recommended that this regimen be used for malaria prophylaxis. They were required to continue taking 1 weekly dose during their stay and for four consecutive weeks after leaving. The recommended weekly dosage for adult patients is 310 mg of the active ingredient [62]. We believe that administering short-term low-dose antimalarial drugs does not result in the development of antibiotic resistance.
Clinical trials of HCQ
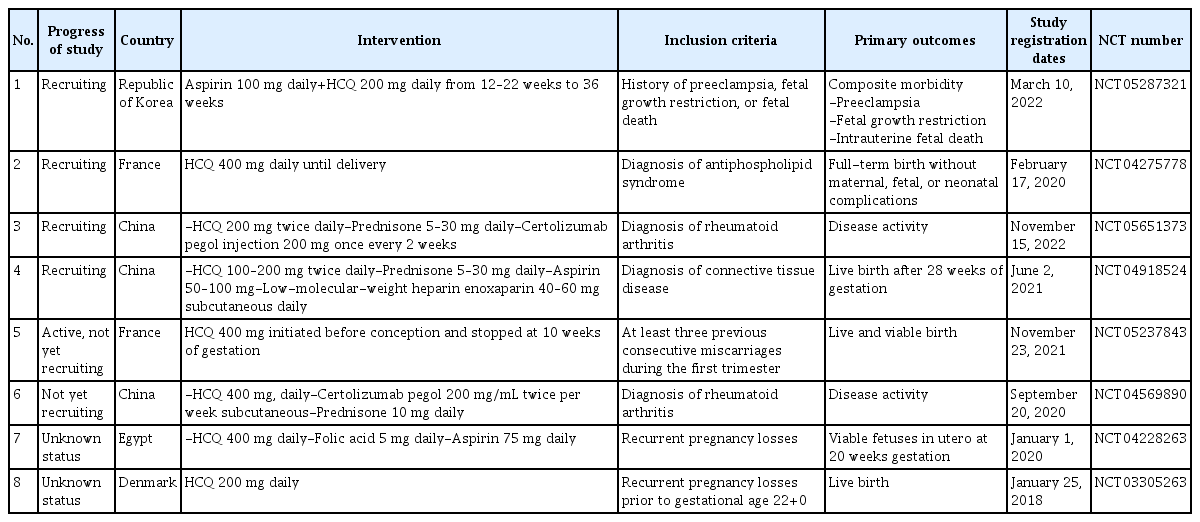
Eight clinical trials (registered at https://clinicaltrials.gov/) have evaluated the effects of HCQ on pregnancy outcomes (Table 1). Four studies focused on the use of HCQ during pregnancy for the treatment of autoimmune diseases including rheumatoid arthritis and antiphospholipid antibody syndrome. Four other studies (studies 1, 5, 7, and 8) focused on the use of HCQ in women with high-risk pregnancies and a history of poor pregnancy outcomes. In a clinical trial conducted in France, the BBQ study (a multicenter, randomized, placebo-controlled, and double-blind study), was designed to evaluate whether HCQ improves the incidence of live births among women with recurrent pregnancy losses. Oral HCQ 400 mg daily was administered from pre-conception to 10 weeks of gestation. The HUGS study is a clinical trial in Korea (a multicenter, single-arm, and open-label study) conducted by our research team since 2022 to evaluate the effects of HCQ on recurrent composite pregnancy complications in women who experienced PE, FGR, and fetal demise. Daily oral 200 mg HCQ with 100 mg aspirin was initiated at 12 weeks of gestation and discontinued at 36 weeks of gestation. All enrolled patients were administered prophylactic low-dose aspirin with HCQ based on the American College of Obstetricians and Gynecologists guidelines, which state that poor pregnancy history is a risk factor for PE. Although previous studies have evaluated the effect of HCQ in pregnancy with autoimmune diseases or recurrent pregnancy loss before the first trimester, to the best of our knowledge, this is the first study to evaluate the use of HCQ during the second and third trimesters in women at risk of placental insufficiency. A prophylactic treatment for placental dysfunction has not yet been established. Therefore, this study has significant clinical relevance in obstetrics.
Conclusion
Placental insufficiency, characterized by recurrence in subsequent pregnancies and complicated by prenatal mortality, is a persistent problem in obstetrics. The lack of prophylaxis except low-dose apsirin are needed to find new preventive treatment of placental insufficiency. The use of HCQ has the potential to reduce pregnancy complications in non-autoimmune women with risk factors for placental insufficiency based on the effect of HCQ on improving pregnancy prognosis in pregnant women with autoimmune diseases. In conclusion, HCQ may be a novel treatment to prevent placental insufficiency-related complications and improve fetal outcomes by inhibiting vascular inflammatory and thrombogenic pathways. Therefore, clinical research on the effects of HCQ in pregnancy should continue to be conducted intensively to prove the clinical effects of HCQ.
Notes
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
None.
Patient consent
None.
Funding information
None.