Fetomaternal outcomes in pregnant women with congenital heart disease: a comparative analysis from an apex institute
Article information
Abstract
Objective
With advancements in cardiac surgical interventions during infancy and childhood, the incidence of maternal congenital heart disease (CHD) is increasing. This retrospective study compared fetal and cardiac outcomes in women with and without CHD, along with a sub-analysis between cyanotic versus non-cyanotic defects and operated versus non-operated cases.
Methods
A 10-year data were retrospectively collected from pregnant women with CHD and a 1:1 ratio of pregnant women without any heart disease. Adverse fetal and cardiac outcomes were noted in both groups. Statistical significance was set at P<0.05.
Results
A total of 86 pregnant women with CHD were studied, with atrial septal defects (29.06%) being the most common. Out of 86 participants, 27 (31.39%) had cyanotic CHD. Around 55% of cases were already operated on for their cardiac defects. Among cardiovascular complications, 5.8% suffered from heart failure, 7.0% had pulmonary arterial hypertension, 8.1% presented in New York Heart Association functional class IV, 9.3% had a need for intensive care unit admission, and one experienced maternal mortality. Adverse fetal outcomes, including operative vaginal delivery, mean duration of hospital stay, fetal growth restriction, preterm birth (<37 weeks), low birth weight (<2,500 g), 5-minute APGAR score <7, and neonatal intensive care unit admissions, were significantly higher in women with CHD than in women without heart disease.
Conclusion
Women with CHD have a higher risk of adverse fetal and cardiac outcomes. The outcome can be improved with proper pre-conceptional optimization of the cardiac condition, good antenatal care, and multidisciplinary team management.
Introduction
Maternal heart disease complicates up to 4% of all pregnancies and is a significant cause of maternal mortality (7.24 per million live births in low- and middle-income countries) after hemorrhage (47%), infection (12%), and hypertensive disorders during pregnancy (7%) [1,2]. We expect an increase in the number of women with heart disease, with better health care and increasing longevity of such women, especially those with congenital heart disease (CHD) [3]. It is difficult for an already-compromised cardiovascular system to sustain the additional risk of normal physiological changes during pregnancy, including increased plasma volume, heart rate, and cardiac output. Hence, these women are more likely to develop heart failure, stroke, arrhythmia, and myocardial infarction during pregnancy [4–7]. Furthermore, pregnancies with CHD are fraught with complications such as preterm labor and increased operative procedures such as instrumental delivery, caesarean delivery, and maternal mortality [8–11]. Neonates born to these mothers also have an increased risk of various adverse outcomes, including small for gestational age, preterm birth, and recurrence of CHD or other congenital anomalies [9,11].
Large cohort studies have reported adverse pregnancy outcomes in patients with heart disease, along with risk assessment [12–14]. Studies specifically conducted in pregnant women with CHD are scarce [15,16]. Moreover, very few studies have compared adverse fetal outcomes in women with CHD with those in women without any heart disease [17,18], the effect of surgery, and the presence or absence of cyanosis [19,20].
Hence, we conducted a retrospective analysis at our institute to compare fetal and cardiac outcomes between pregnant women with CHD and those without heart disease. A subgroup analysis was also performed between antenatal women with cyanotic and non-cyanotic CHD and those with operated and non-operated heart defects.
Materials and methods
This retrospective study was conducted at the Department of Obstetrics and Gynecology of a tertiary care hospital. Data was collected for 10 years, from August 2013 to July 2023, for all pregnant women with CHD who were enrolled under two units and delivered at our institute during this period. To compare outcomes, pregnant women without any heart disease who delivered at our institute during the same period were also included at a ratio of 1:1. Ethical clearance was obtained from the Institute Ethics Committee (IEC- 581/06.10.2023). Patients with missing or insufficient information were excluded. All relevant information, including demographic profile, obstetric history, details of the current pregnancy, delivery details, and information related to the newborn, were noted. Information regarding heart disease included signs and symptoms at presentation; New York Health Association Functional class (NYHA) at presentation; cyanotic or acyanotic nature of the disease; cardiac drugs; investigations including chest radiography, electrocardiography, and echocardiography; history of cardiac surgery; and length of hospital stay. The Modified World Health Organization [21] classification was used to grade the severity of the heart disease. The outcome measures considered were maternal cardiac outcomes, including cardiac failure, arrhythmias, thromboembolic events, pulmonary arterial hypertension (PAH), need for intensive care unit (ICU) admission, and maternal mortality. Maternal obstetric outcomes, including preeclampsia/eclampsia (PE/E), gestational diabetes mellitus (GDM), placenta previa, abruption, antepartum hemorrhage (APH), mode of delivery, period of gestation (POG) at delivery, peripartum or postpartum hemorrhage, and postpartum complications (infection, wound dehiscence, and sepsis), were collected. Among fetal outcomes, fetal growth restriction (FGR), prematurity (>37 weeks), birth weight, low birth weight (LBW) (<2,500 g), APGAR score less than 7 at 5 minutes of birth, stillbirth, congenital heart defect or another non-cardiac congenital anomaly in the newborn, need for neonatal intensive care unit (NICU) admission, and neonatal mortality were assessed.
Data analysis was performed using the statistical software SPSS IBM version 26.0 (IBM Corp., Armonk, NY, USA). The normality assumptions of the continuous variables were assessed using the Kolmogorov-Smirnov test. Descriptive statistics, including mean and standard deviation, were calculated for data that followed a normal distribution. Statistical analysis involved the application of the student’s t-test to compare the mean values of the two groups. Categorical data were displayed using frequency and percentage values. Frequency data across categories were analyzed using the chi-square or Fisher’s exact test. The study provided unadjusted odds ratios (OR) with 95% confidence intervals for all 2×2 tables. A two-tailed probability with a value of P<0.05 was considered statistically significant.
Results
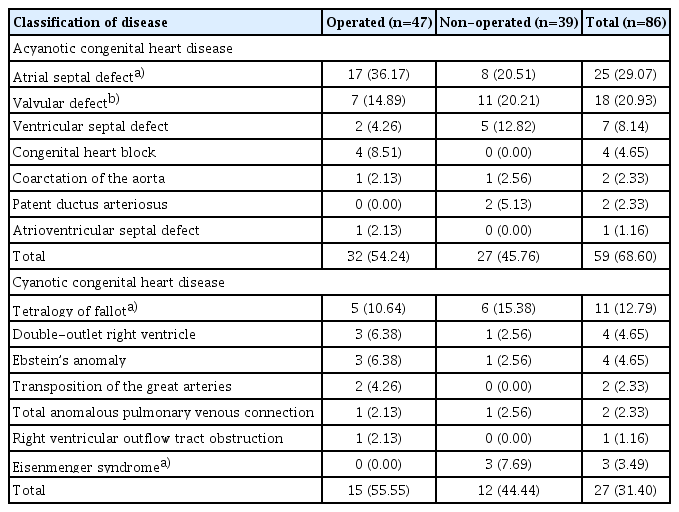
A total of 86 pregnant women with CHD delivered at our institute during this period. The most common type of CHD was atrial septal defect (ASD), accounting for 29.07%. Pregnant women with acyanotic defects was 68.60% and 31.40% had cyanotic CHD. Out of 86, corrective surgery was already done in 55% of cases (Table 1).
1. Comparative analysis between pregnant women with CHD (CHD group) and those without any heart disease (non-cardiac group)
The pregnant women with CHD presented at a significantly earlier age (26.28±4.373 years) compared to those without heart disease (28.49±4.189 years) (P=0.001), whereas parity was comparable between these two groups.
Among the obstetric outcomes, operative vaginal deliveries were significantly higher in the CHD group than in the noncardiac group. There were no significant differences in the development of GDM, PE/E, APH, caesarean delivery, and postpartum complications, including sepsis, wound infection, and postpartum hemorrhage. However, there was a significantly longer hospital stay in the CHD group compared to those without heart disease (Table 2).
The odds of FGR, preterm birth, neonates with LBW, 5-minute- APGAR score <7, rate of NICU admission, and congenital anomalies in newborns were all significantly higher in the CHD group than in the non-cardiac group. Neonatal mortality was not observed in any group. There was one stillbirth in the CHD group; the patient had corrected tetralogy of fallot (TOF) and was referred to our hospital for congestive heart failure (CHF) with NYHA functional class IV in the third trimester, the acute episode was managed conservatively, and labor was induced at 37 weeks and 2 days in view of FGR, fetal distress was noted in the second stage of labor, forceps application was performed, but the fetus was delivered as a stillbirth.
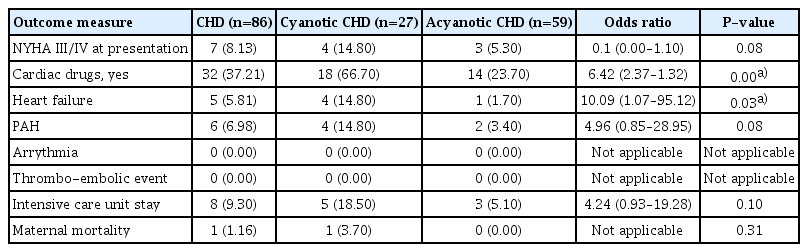
Among adverse cardiovascular outcomes in the CHD group, 8.13% presented with NYHA functional class IV, 5.81% suffered from heart failure, 6.98% had PAH, and 9.30% had a need for ICU admission. There was no incidence of arrhythmia or other thromboembolic events in any group (Table 3). There was one case of maternal mortality in the CHD group; the patient was a primigravida diagnosed with uncorrected TOF and moderate PAH. Preterm caesarean delivery was performed when the patient developed CHF; however, the patient died on the same day due to cardiac failure.
2. Subgroup analysis within the CHD group
A subgroup analysis was performed between pregnant women with cyanotic versus acyanotic CHD. Out of 86 women, 31.40% had cyanotic CHD and 68.60% suffered from acyanotic CHD. The average age in the cyanotic and acyanotic groups was 27.26±4.974 years and 25.83±4.035 years, respectively, and it was comparable (P=0.161). A higher number of patients in the cyanotic group presented with NYHA class III/IV and developed heart failure (Table 3). The mean duration of hospital stay was significantly longer in the cyanotic group than in the acyanotic group (P=0.004) (Table 4).
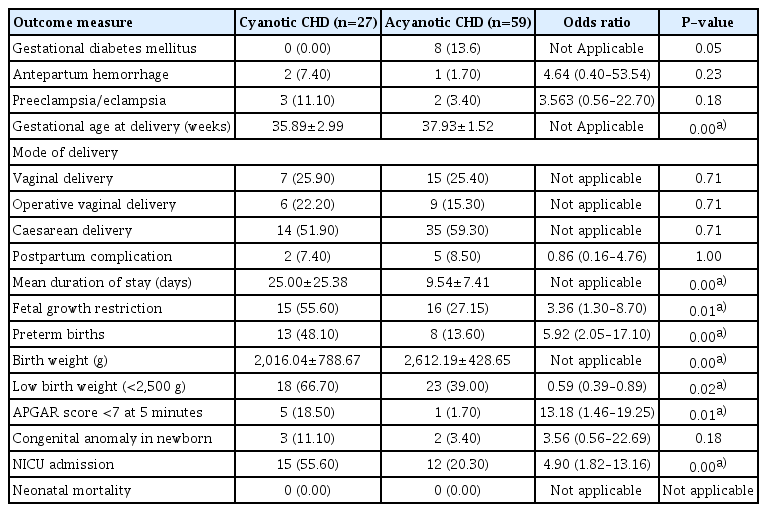
On comparing the obstetrical outcomes (Table 4), the incidence of GDM, PE/E, APH, number of caesarean deliveries, and postpartum complications were comparable in both groups. Among the fetal outcomes, the mean birth weight was significantly lower in the cyanotic group. Adverse fetal outcomes, including FGR, prematurity, LBW, and NICU admission, were higher in the cyanotic group than in the acyanotic group (Table 4).
To perform a subgroup analysis based on corrective surgery, the patients were divided into two groups: operated and non-operated CHD groups (Table 5). The average age in years was comparable between the operated and nonoperated groups, (26.36±4.125 years vs. 26.18±4.707 years, respectively; P=0.849). The mean duration of hospital stay was significantly longer in the non-operated CHD group than in the operated CHD group. In the non-operated group, 27 patients were acyanotic and 12 were cyanotic; the majority did not require any surgery, including eight with ASD, five with ventricular septal defect, and 11 with valvular defects. However, three developed Eisenmenger syndrome, including two non-operated ASD patients and one non-operated TOF patient, who also suffered from cardiac complications, including PAH and heart failure, although this was not statistically significant. The obstetric and fetal outcomes in both groups were comparable. There were no significant differences in the development of GDM, PE, APH, and postpartum complications, delivery by caesarean section, POG at delivery, and FGR.
Discussion
With advancements in modern imaging and surgical capabilities, the survival of CHD patients has improved dramatically [22]. Women with complex CHD can reach childbearing age and pregnancy, and it is more challenging to manage these pregnancies by treating clinicians. In our study, we found that pregnant women with CHD had more adverse fetomaternal and cardiac outcomes, and subgroup analysis showed that adverse outcomes were significantly higher in the cyanotic group than in the acyanotic CHD group; however, we did not find any statistically significant difference between the operated versus non-operated CHD groups.
ASD was the most common (29%) type of CHD observed in the present study, which is similar to previous studies by Ramlakhan et al. [15] and Liu et al. [18]. Pregnant women with CHD presented at a significantly earlier age than those without heart disease. This observation was in accordance with the findings of Ramlakhan et al. [15] but contrary to other studies [4,5,17]. The awareness of their condition and continuous interaction with treating cardiologists might be the reason for this observation. The need for more observation during the postpartum period prolongs the hospital stay of heart disease patients, as recorded by the majority of researchers, and ultimately leads to an increased financial burden on healthcare facilities [16,18].
Most women tolerated pregnancy well with regard to cardiovascular complications. A heart failure rate of 6.6% and maternal mortality rate of 0.3% were observed by Ramlakhan et al. [15] in their study, consistent to our findings. Opotowsky et al. [4] observed that cardiovascular events were more common in pregnant women with CHD with an OR of 8.4 than in those without CHD.
In our study, adverse fetal outcomes, including hypertension during pregnancy (4.1% vs. 5.8%), and caesarean delivery (46.3% vs. 57.05%), APGAR score <7 at 5 minutes (7.0% vs. 5.9%), fetal CHD (2.3% vs. 3.5%) and non-cardiac congenital conditions (3.5% vs. 5.9%) were comparable to the observations of Ramlakhan et al. [15] whereas, the incidence of stillbirths (1.2% vs. 0.5%), preterm delivery (25.5% vs. 16.0%), and FGR (36.2% vs. 10.3%) was higher. Being a referral center, majority of patients presented to us with advanced gestational age, which makes it difficult to optimize the cardiac and obstetric conditions, hence the preterm deliveries were significantly higher at our center compared to the global average of 10.6% [23]. The opportunity for preconceptional optimization of the cardiac condition was also missed, as we received most patients after getting pregnant. There is a need for pre-conceptional optimization of the medical condition and timely referral to an expert obstetrician, which is possible with the help of the treating cardiologist and creating awareness about the condition.
Eisenmenger syndrome is considered an absolute contraindication to pregnancy because a reduction in systemic vascular resistance accelerates the progression of the right-to-left shunt, leading to more cyanotic spells and a higher chance of paradoxical emboli. In our study, three patients had Eisenmenger syndrome, two had an unrepaired ASD as the primary defect and one had an unrepaired TOF. All patients who developed adverse cardiac outcomes, including PAH and CHF, had preterm delivery with growth-restricted fetuses along with Doppler changes; the ICU stay of the mother and NICU stay of the newborn were also there. Similarly, our subgroup analysis showed that patients with cyanotic heart disease had significantly higher odds of adverse fetomaternal and cardiovascular outcomes compared to the acyanotic group. Our findings corroborate those of Ladouceur et al. [20] who observed a higher risk of heart failure, deterioration of NYHA functional class, and arrhythmias in their multicenter study.
In our subanalysis on the basis of surgery, there were 55% of patients in the operated CHD group and 45% of patients in the non-operated CHD group, whereas the study by Ramlakhan et al. [15] had almost 2/3rd and 1/3rd distribution for the same. Comparable outcomes were observed between the operated and non-operated groups, including pregnancy-induced hypertension, caesarean delivery rate, preterm birth, low APGAR score, and FGR, similar to the observation in Ramlakhan et al. [15]. These results were also consistent with the findings of Yadav et al. [24], who compared the outcomes between operated and non-operated patients with CHD during pregnancy. Furthermore, in the operated CHD group, we observed a maternal cardiac complication rate of 6–8%, consistent with the 8% rate by Takatsuki et al. [19], who also highlighted the need for more caution in the management of NYHA functional class II repaired cyanotic CHD patients, as they appeared stable but had high chances of adverse events. Single maternal mortality in our study was also presented in the same manner. Similar to their observation of 5.2% of infants with CHD, we also had 4.3% of newborns with CHD in the corrected CHD group.
Our study represents a large data of 10 years duration from a premier referral institute that makes a significant contribution from India. A subanalysis based on corrective surgery and the presence or absence of cyanosis adds valuable information about the subject. The retrospective nature of the study is the main limitation, as we had a significant chance of losing important information. Being collected from an apex referral center also reduces the generalizability of the outcomes. The main focus was on the delivery period; adverse events such as miscarriage and other significant information during the antenatal period were not included in the study.
Congenital heart disease has a myriad spectrum of lesions, each with its own implications and treatments. The outcome is dependent upon many factors, such as the type of lesion, status of the lesion, center-specific care, infrastructure and availability of multidisciplinary care, time of presentation, and patient compliance to antenatal care. However, these conditions require a multidisciplinary approach from the very beginning. With pre-conceptional optimization of the cardiac condition, correction of the cardiac lesion before pregnancy, timely referral to the appropriate center, good antenatal care, and multidisciplinary team management, we can expect better outcomes for these patients.
Notes
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
The study was approved by the Institute Ethics Committee of All India Institute of Medical Sciences, New Delhi (Approval no. IEC-581/06.10.2023).
Patient consent
Not required as it is a retrospective study.
Funding information
None.