 |
 |
- Search
| Obstet Gynecol Sci > Volume 57(6); 2014 > Article |
Abstract
Lymphoma, especially non-Hodgkin's lymphoma is extremely rare in pregnancy. A 24-year-old pregnant woman was diagnosed with diffuse large B-cell lymphoma (DLBCL), a subgroup of non-Hodgkin's lymphoma, at 24 weeks' gestation, and was treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy. After 4 cycles of R-CHOP, she delivered a healthy baby via cesarean section at 34 weeks and 5 days' gestation because of preterm contraction-related fetal distress. The patient was administered the remaining 2 cycles of R-CHOP after delivery. Follow-up magnetic resonance imaging and computed tomography showed complete remission. Here, we report a rare case of DLBCL successfully treated with R-CHOP chemotherapy during pregnancy, we also performed a systematic review of literature for similar cases. There were 3 earlier reports of R-CHOP treatment for DLBCL. All cases, including our case, resulted in preterm birth. Together, these findings suggest that R-CHOP chemotherapy for DLBCL in pregnancy may be associated with preterm birth.
Unexpected diagnosis of cancer, especially extremely rare hematological cancer like non-Hodgkin's lymphoma (NHL) during pregnancy is a concern to the patient, family and obstetrician. In our systematic review, we identified 3 earlier reports of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy for diffuse large B-cell lymphoma (DLBCL) during pregnancy. All these case studies were described by hematologists, and they did not focus on the pregnancy outcome. Here, we report a rare case of DLBCL successfully treated with R-CHOP chemotherapy during pregnancy and associated with preterm birth. We also performed a systematic review of literature for similar cases.
A 24-year-old pregnant woman (gravida 1, para 0) whose medical and familial histories were unremarkable, presented with sore throat and a palpable mass in the right submandibular area. Neck biopsy of the submandibular mass revealed DCBCL, a subgroup of NHL.
She was at 22 weeks' gestation at the time of diagnosis and was recommended for termination of pregnancy at a different hospital. But we explained the risk of chemotherapy in pregnancy to the patient and her family before chemotherapy and the patient and her family agreed to receive the chemotherapy during pregnancy. Subsequently, the patient underwent neck and abdominal magnetic resonance imaging (MRI) (Fig. 1). A 2.4×1.7-cm enlarged lymph node at the right level IIA (Fig. 1A) and a 5.4×2.5-cm mass in the right palatine tonsil (Fig. 1C) were identified in neck MRI images. Initial blood tests revealed normal blood counts and chemistry, including a normal beta-microglobulin level of 1.16 mg/L. The fetal weight was estimated to be approximately 1,582 g at 29 weeks' gestation on ultrasonography. The fetus showed no abnormal findings. The patient was treated with R-CHOP chemotherapy immediately after evaluation for reproductive toxicology and receiving approval from the hematologists and the patient. The patient and fetus were closely monitored before and after induction of chemotherapy.
The patient received the standard dose of R-CHOP every 3 weeks: 375 mg/m2 rituximab, 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine, and 100 mg/day prednisone on days 1 to 5. A total of 6 cycles were planned. On the first day of chemotherapy, the patient complained of mild skin rash following rituximab infusion, which resolved following an injection of hydrocortisone. After the second cycle of chemotherapy, she had preterm contractions, which resolved following treatment with tocolytics (magnesium sulfate) for several days. Follow-up neck MRI showed marked reduction and almost complete disappearance of homogenous masses both in the neck (Fig. 1B) and right tonsil (Fig. 1D).
After 4 cycles of R-CHOP, she was admitted to the hospital with preterm contraction at 33 weeks and 2 days' gestation. Nitrazine and premature rupture of membrane tests were negative, and the fetal estimated weight and other fetal recordings were within normal range. Emergency cesarean section was performed at 34 weeks and 5 days' gestation because of fetal distress (decreased variability:recurrent variable deceleration).
A 2,560 g, healthy baby girl was born with an APGAR (activity, pulse, grimace, appearance, and respiration) score of 7/9 without any complications. Echocardiography, brain ultrasonography, blood tests, whole body radiography were performed on the infant on the second day. Echocardiography showed a 1.2 mm patent ductus arteriosus, which was closed the following day. All other test results were unremarkable.
After delivery, the patient was administered the remaining 2 cycles of R-CHOP therapy. The follow-up computed tomography and, positron emission tomography/computed tomography scans showed complete remission. At 13 months after delivery, the patient showed no evidence of lymphoma, and her child weighed over 10 kg and did not show any developmental delays or physical abnormalities.
Obstetricians are often reluctant to administer chemotherapeutic agents during pregnancy because of their relatively low molecular weight, which allows them to cross the human placenta. The teratogenicity of these chemotherapeutic agents largely depends on the timing of exposure and the kinetics involved in placental transfer. Chemotherapy during first trimester of pregnancy may increase the risk of spontaneous abortions, fetal death, and major malformations, as chemotherapy could interfere with organogenesis [1,2]. The main embryonic period associated with major anomalies in heart, neural tube, and limbs is between 5 and 10 weeks' gestation. After 10 weeks' gestation, functional defects or minor anomalies can occur in the palate and ear.
A recent case report described a patient who was diagnosed with primary mediastinal large B-cell lymphoma (a rare variant of DLBCL) at 12 weeks' gestation and received R-CHOP therapy from 13 to 31 weeks' gestation. The patient delivered a healthy infant at 34 weeks and 4 days' gestation prematurely [3].
Although exposure to chemotherapeutic agents in the second and third trimesters is not associated with malformations, an increase in the risk of stillbirth, fetal growth restriction, preterm delivery, and low birth weight is possible. Exposure to chemotherapeutic agents in the second and third trimesters is considered relatively safe for the patient and fetus compared to exposure in the first trimester [2]. In our analysis of the 4 cases including our case, there were no major complications except for preterm birth.
Lymphomas are rare during pregnancy and NHL during pregnancy is extremely rare. If the treatment cannot be delayed until delivery, chemotherapy can be considered. The CHOP regimen has been commonly used in NHL and is considered safe in the second and third trimesters [4,5]. An earlier study had reported that R-CHOP shows better prognosis than CHOP therapy in young non-pregnant DLBCL patients [6]. Considering that most pregnant patients are in the young age group, R-CHOP chemotherapy is more efficient during pregnancy if rituximab is not harmful to the fetus. Rituximab is a monoclonal antibody directed against B-cell surface antigen CD20, which can cross the placenta. According to a previous report of 231 pregnancies with preconceptional or antepartum exposure to rituximab, most pregnancies resulted in uncomplicated live births. Two congenital malformations (2.2%; clubfoot in a twin, and cardiac malformation in a singleton birth) were reported [7], and it was similar to the risk of general population. It is remarkable that the preterm delivery rate (19%) [7] was higher than that in the general population (10% to 12%) [8]. It was concluded that rituximab is relatively safe during pregnancy [7,9].
A systematic review of the literature identified 3 cases of R-CHOP chemotherapy for DLBCL during pregnancy, with successful deliveries (Table 1) [3,10,11]. The first case of a pregnant patient with DLBCL treated successfully with R-CHOP was published at 2006. The patient was at 15 weeks' gestation at the diagnosis, and delivered a healthy baby at 33 weeks' gestation [10]. The second case was a pregnant patient who was diagnosed with primary mediastinal large B-cell lymphoma at 22 weeks' gestation. She was treated with R-CHOP successfully and delivered a healthy baby by cesarean section [11]. Remarkably, all 4 cases, including our case, resulted in preterm birth. Our patient was treated with R-CHOP and the infant was born at 34 weeks and 5 days' gestation prematurely. Together these findings suggest that R-CHOP chemotherapy during pregnancy for DLBCL may be associated with preterm birth.
In summary, a patient with DLBCL at 22 weeks' gestation was successfully treated with R-CHOP chemotherapy, and underwent successful delivery through cesarean section. The mother and the baby were in good health; however, the baby was born prematurely. We believe that although R-CHOP chemotherapy may be associated with preterm birth, it is a relatively safe option for the treatment of DLBCL during pregnancy.
Acknowledgments
This study was supported by grant no. K1220732 from the Korea University College of Medicine.
References
1. Leslie KK, Koil C, Rayburn WF. Chemotherapeutic drugs in pregnancy. Obstet Gynecol Clin North Am 2005;32:627-640. PMID: 16310676.


2. Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Fetal outcome after in utero exposure to cancer chemotherapy. Arch Intern Med 1992;152:573-576. PMID: 1546920.


3. Perez CA, Amin J, Aguina LM, Cioffi-Lavina M, Santos ES. Primary mediastinal large B-cell lymphoma during pregnancy. Case Rep Hematol 2012;2012:197347PMID: 23198190.



4. Vandenbriele C, Dierickx D, Amant F, Delforge M. The treatment of hematologic malignancies in pregnancy. Facts Views Vis Obgyn 2010;2:74-87. PMID: 25302102.


5. Pereg D, Koren G, Lishner M. The treatment of Hodgkin's and non-Hodgkin's lymphoma in pregnancy. Haematologica 2007;92:1230-1237. PMID: 17666365.


6. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379-391. PMID: 16648042.


7. Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011;117:1499-1506. PMID: 21098742.


8. Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med 2010;362:529-535. PMID: 20147718.


9. Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy. Lancet 2012;379:580-587. PMID: 22325663.


10. Decker M, Rothermundt C, Hollander G, Tichelli A, Rochlitz C. Rituximab plus CHOP for treatment of diffuse large B-cell lymphoma during second trimester of pregnancy. Lancet Oncol 2006;7:693-694. PMID: 16887487.


11. Kim EK, Kim SJ, Park SB, Cho JH, Ko YH, Kim WS. Rituximab plus CHOP for the treatment of primary mediastinal large b cell lymphoma in a pregnant woman. Korean J Med 2011;80:S273-S277.
Fig. 1
(A) A 2.4×1.7-cm lymph node (arrow) in the neck (level IIa), T2 magnetic resonance imaging (MRI). (B) After 4 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy, no masses are seen, T2 MRI. (C) A 5.4×2.5-cm mass (arrow) in the right palatine tonsil, T1 MRI. (D) After 4 cycles of R-CHOP chemotherapy, no masses are seen, T1 MRI.
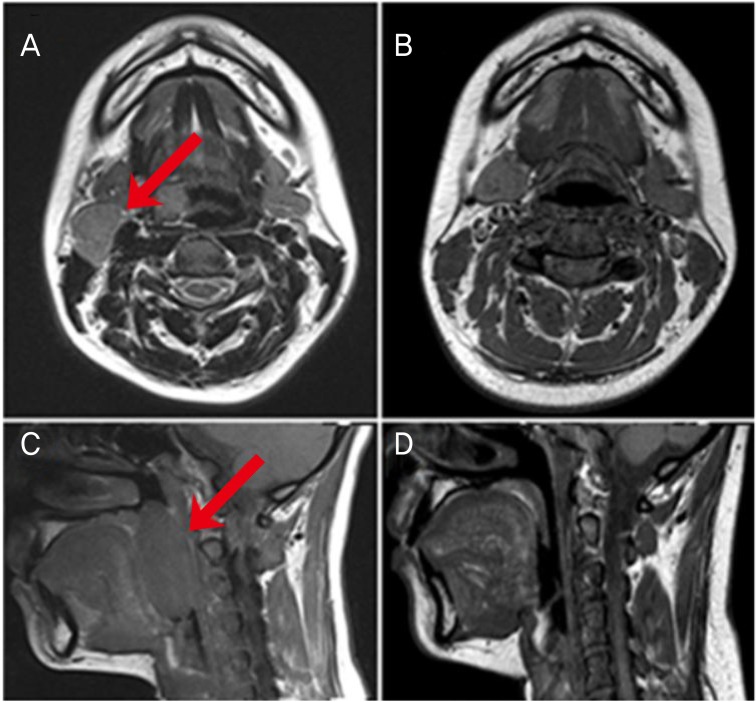
Table 1
Case reports and systematic review
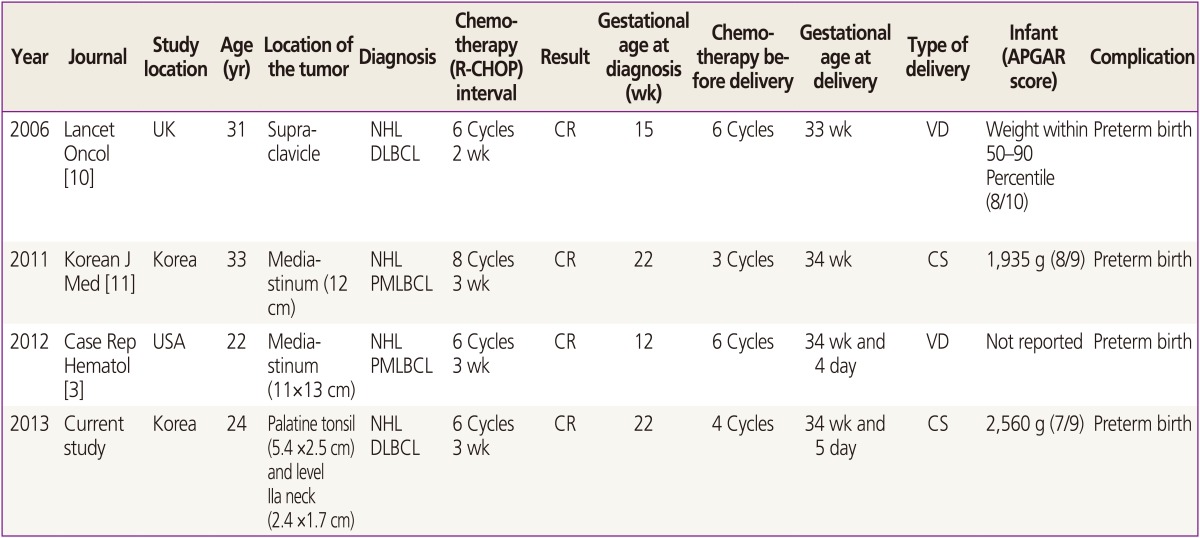
R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; APGAR, activity, pulse, grimace, appearance, and respiration; NHL, non-Hodgkin's lymphoma; DLBCL, diffuse large B-cell lymphoma; CR, complete remission; VD, vaginal delivery; PMLBCL, primary mediastinal large B-cell lymphoma (a variant of DLBCL); CS, cesarean section.
-
METRICS

-
- 21 Crossref
- 3,087 View
- 40 Download
- Related articles in Obstet Gynecol Sci



















