 |
 |
- Search
| Obstet Gynecol Sci > Volume 57(6); 2014 > Article |
Abstract
Cervical cancer is one of the most common malignancy diagnosed during pregnancy. The experience of the use of neoadjuvant chemotherapy (NACT) with paclitaxel plus cisplatin during pregnancy is limited. Three pregnant women with International Federation of Gynecology and Obstetrics (FIGO) stage IB cervical cancer received NACT with paclitaxel plus cisplatin until fetal lung maturity, and then underwent cesarean delivery and radical hysterectomy. Two of our patients had intermediate pathologic risk factors, and received adjuvant chemotherapy with the same regimen used in NACT. All patients did not have any evidence of disease recurrence for follow-up of 3, 4, and 8 years, respectively. NACT with paclitaxel plus cisplatin followed by radical hysterectomy and adjuvant chemotherapy could be considered as one of feasible alternatives to primary radical surgery or concurrent chemoradiation therapy with the termination of pregnancy in pregnant women with FIGO stage IB cervical cancer who have two or more intermediate pathologic-risk factors.
Cervical cancer is one of the most common malignancies diagnosed during pregnancy with a reported incidence ranging between 1 and 12 per 10,000 pregnancies [1]. Owing to its low incidence, there is no specific treatment guideline for this disease. The management is influenced mostly by gestational age, the International Federation of Gynecology and Obstetrics (FIGO) stage of disease, and the patient's desire to maintain pregnancy [2].
It has been reported that neoadjuvant chemotherapy (NACT) followed by cesarean delivery and radical hysterectomy (RH) could be one of feasible treatment options in selected patients with FIGO stage IB cervical cancer, who were diagnosed in the second trimester of pregnancy and wanted to maintain their pregnancies [2]. Since the mid-1990s, NACT with single-agent cisplatin or combination of cisplatin plus vincristine and bleomycin has been used most often. Recently, NACT with paclitaxel plus cisplatin has been used in non-pregnant cervical cancer patients with response rates between 40% and 50% [3]. Several investigators also reported the favorable obstetric outcome of 8 pregnant women with cervical cancer treated by NACT with paclitaxel plus cisplatin [4,5,6,7]. We report three pregnant women with FIGO stage IB cervical cancer who were treated by NACT with paclitaxel plus cisplatin, followed by cesarean section, RH and postoperative chemotherapy only.
A 31-year-old woman at 19+0 weeks of gestation was referred to our institution for evaluation of cervical mass. A cervical biopsy revealed a moderately differentiated mucinous adenocarcinoma. Magnetic resonance imaging (MRI) confirmed a 2.8-cm-sized cervical mass without vaginal and parametrial involvement. The patient was diagnosed to have FIGO stage IB1 disease. After comprehensive counseling, the written informed consent and institutional review board approval were obtained to start NACT. NACT consisting of paclitaxel 135 mg/m2 plus cisplatin 60 mg/m2 every 3 weeks was given at 22 weeks' gestation. Two additional NACT was administered at 25 and 28 weeks, respectively. However, the patient refused additional NACT and wanted to schedule the delivery of her baby. Fetal well-being was shown by ultrasonographic evaluation before surgery. In addition, fetal pulmonary maturity was confirmed by amniocentesis and the concentration of lecithin is above twice that of spingomyelin. She underwent cesarean section at 33+0 weeks' gestation, and then followed by type III RH with pelvic and para-aortic lymphadenectomy. Final histology revealed a 1.0 cm sized mucinous adenocarcinoma without deep stromal invasion (DSI). Although lymph-vascular space invasion (LVSI) was identified, there was no tumor infiltration to the adjacent vaginal cuff and parametrium and all 26 lymph nodes harvested were tumor negative. Eight years later, the patient did not show any evidence of disease recurrence, and her child is developing normally.
A 26-year-old woman presented an invasive squamous cell carcinoma (SCC) of the cervix, 2 cm in diameter, diagnosed at 25+0 weeks of gestation. She was diagnosed to have FIGO stage IB1 disease. We recommended NACT, but she refused the subsequent treatment and disappeared from our service. Five weeks later, she revisited our institution complaining of vaginal bleeding for several weeks. The cervical mass was increased up to 3.5 cm in size and suspicious for left parametrial involvement on MRI. After one cycle of NACT using paclitaxel plus cisplatin at 30 weeks of pregnancy, she underwent cesarean section and radical surgery at 34+6 weeks' gestation because administration of chemotherapy within 3 weeks of anticipated delivery or beyond 35 weeks of gestation is not recommended [8]. Fetal well-being was assessed before surgery and after each cycle of chemotherapy with ultrasound including non stress test. The histopathologic examination demonstrated a 3.0-cm-sized keratinizing large-cell SCC with clear vaginal resection margins and negative lymph nodes. The tumor showed LVSI, but no infiltration of the parametrium. She was offered adjuvant chemotherapy due to two intermediate risk factors such as LVSI and DSI. Adjuvant chemotherapy as above was initiated at 13 days after surgery. She received three cycles of postoperative chemotherapy every 3 weeks. The patient did not have any evidence of disease recurrence for follow-up of 4 years. The newborn infant showed no sign of any neuropsychomotor abnormalities.
A 38-year-old woman at 13+0 weeks of gestation presented with high-grade squamous intraepithelial lesion found by a Pap smear. Colposcopy and MRI showed an exophytic cervical mass measuring 5 cm in greatest dimension (Fig. 1). A subsequent cervical biopsy revealed a SCC. The patient was diagnosed as FIGO stage IB2 cervical cancer. She was started on NACT using paclitaxel plus cisplatin at 18 weeks' gestation. After the fourth cycles of NACT, the tumor size decreased from 5.4 to 2.5 cm on MRI (Fig. 2A). Fetal well-being was assessed before and after each treatment with ultrasound including non stress test. Cesarean section followed by radical surgery was performed at 35+2 weeks' gestation (Fig. 2B). Microscopically, the tumor had a non-keratinizing large-cell SCC characterized by nested tumor cells with pleomorphic nuclei and frequent mitotic figures, and an absence of keratin pearl formation, with stromal invasion (Fig. 2C). The histopathologic report revealed a 2.5-cm-sized cervical mass with clear vaginal resection margins and negative lymph nodes. Neither LVSI nor parametrial involvement was found. Adjuvant chemotherapy with the same regimen as above was initiated at 8 days after surgery because of two intermediate-risk factors such as larger tumor size and DSI. She received two cycles of postoperative chemotherapy every 3 weeks. After 3 years of the operation, the patient had no evidence of local or distant relapse, and her infant demonstrates normal neuropsychomotor development.
In cases in which fetal viability is attainable, the most common approach to pregnant women with cervical cancer is to carefully follow up patients clinically and radiologically and to delay treatment of disease, particularly in patients with early-stage disease and no nodal involvement [9]. Karam et al. [10] suggested that the risk of clinically significant disease progression was small for very early-stage disease (stage IA and small IB1), and most patients with FIGO stage IB disease have fared well with planned delay in therapy. As shown by the present report, however, one of our patients (case 2) showed a significant increase in tumor volume from 2 to 3.5 cm during the 5 weeks of observation, so planned treatment delay needs to be deliberately considered. NACT with the preservation of the pregnancy, therefore, may be considered as one of feasible alternatives to other treatment modalities in pregnant women with FIGO stage IB cervical cancer.
To date, there have been 11 cases of pregnant women (including the 3 cases added by present report) with stage IB to IIB cervical cancer treated with NACT using paclitaxel plus cisplatin followed by cesarean section and RH in the literature (Table 1) [4,5,6,7]. Most patients have shown significant tumor regression, and RH was feasible at the time of delivery following documentation of fetal lung maturity at 32 and 34 weeks of gestation. However, one patient died of disease. Among 9 patients with stage IB disease, only 1 patient with a small cell neuroendocerine carcinoma who did not postoperative adjuvant therapy developed widespread metastases and died 24 months after diagnosis (Table 1). Fruscio et al. [7] retrospectively reviewed 27 cases of pregnant women with FIGO stage IB1-IIIB cervical cancer treated with NACT. In particular, among 13 patients with stage IB2 disease, 5 patients, who received NACT with cisplatin alone or cisplatin plus vincristine and bleomycin regimen followed by RH, died from recurrent disease [7,11,12]. However, among 5 patients with stage IB2 disease who received NACT with paclitaxel plus cisplatin regimen followed by RH, none had recurrent disease [5,6,7]. Thus, NACT with paclitaxel plus cisplatin followed by RH for FIGO stage IB with tumor size Ōēź2 cm during pregnancy has resulted in both favorable oncologic and obstetric outcomes.
Chemotherapy given during the second and third trimesters is associated with intrauterine growth retardation, prematurity, and low birth weight in about one half of exposed infants [8]. Zemlickis et al. [13] also recommended using lower doses of chemotherapy during pregnancy because of higher concentration of free chemotherapeutic agent in pregnant woman compared with women who were not pregnant. In the current study, NACT with reduced dose of cisplatin (60 mg/m2) and paclitaxel (135 mg/m2) every 3 weeks was used. In addition to the dose reduction, NACT within 3 weeks of anticipated delivery or beyond 35 weeks of gestation was not administered to avoid transient neonatal myelosuppression and potential complications such as bleeding, sepsis, and death at the time of delivery [8].
Although the intermediate-risk group has a favorable prognosis (85% to 90% survival rate) without adjuvant treatment, several studies have shown that intermediate risk factors increase the risk of recurrence to 31% in patients, particularly with multiple intermediate risk factors, suggesting the need for adjuvant radiotherapy [14]. In addition, some data have suggested that the adjuvant therapeutic effects of both chemotherapy and radiotherapy were similar, without increasing severe postsurgical complications [15]. In the present study, two of our patients (cases 2 and 3) had intermediate risk factors, and received adjuvant chemotherapy with the same regimen used in NACT. Among 29 patients in other studies, 3 patients showed intermediate pathologic-risk factors. Two patients who received adjuvant therapy remain free of disease. However, one patient refused adjuvant treatment and she had a 7 cm recurrent tumor in the left pelvic cavity. Thus, the administration of adjuvant chemotherapy in this study might be justified in early-stage cervical patients, particularly sexually active premenopausal women who have intermediate risk factors. All patients treated radical surgery plus postoperative chemotherapy reported normal sexual activity.
In conclusion, NACT with paclitaxel plus cisplatin followed by radical surgery and postoperative chemotherapy could be considered as one of feasible alternatives to primary surgery or concurrent chemoradiation therapy with the termination of pregnancy in pregnant women with FIGO stage IB cervical cancer. However, further studies are required to determine the optimal treatment regimens in pregnant patients with cervical cancer. Also, a longer follow-up is needed to evaluate the safe outcome of these children.
References
2. Hunter MI, Tewari K, Monk BJ. Cervical neoplasia in pregnancy. Part 2: current treatment of invasive disease. Am J Obstet Gynecol 2008;199:10-18. PMID: 18585521.


3. Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 1999;17:2676-2680. PMID: 10561341.


4. Palaia I, Pernice M, Graziano M, Bellati F, Panici PB. Neoadjuvant chemotherapy plus radical surgery in locally advanced cervical cancer during pregnancy: a case report. Am J Obstet Gynecol 2007;197:e5-e6. PMID: 17904952.

5. Chun KC, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Neoadjuvant chemotherapy with paclitaxel plus platinum followed by radical surgery in early cervical cancer during pregnancy: three case reports. Jpn J Clin Oncol 2010;40:694-698. PMID: 20427545.


6. Li J, Wang LJ, Zhang BZ, Peng YP, Lin ZQ. Neoadjuvant chemotherapy with paclitaxel plus platinum for invasive cervical cancer in pregnancy: two case report and literature review. Arch Gynecol Obstet 2011;284:779-783. PMID: 21691768.


7. Fruscio R, Villa A, Chiari S, Vergani P, Ceppi L, Dell'Orto F, et al. Delivery delay with neoadjuvant chemotherapy for cervical cancer patients during pregnancy: a series of nine cases and literature review. Gynecol Oncol 2012;126:192-197. PMID: 22555106.


8. Brewer M, Kueck A, Runowicz CD. Chemotherapy in pregnancy. Clin Obstet Gynecol 2011;54:602-618. PMID: 22031250.


9. Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet 2012;379:558-569. PMID: 22325661.


10. Karam A, Feldman N, Holschneider CH. Neoadjuvant cisplatin and radical cesarean hysterectomy for cervical cancer in pregnancy. Nat Clin Pract Oncol 2007;4:375-380. PMID: 17534393.


11. Lai CH, Hsueh S, Chang TC, Tseng CJ, Huang KG, Chou HH, et al. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol 1997;64:456-462. PMID: 9062150.


12. Rabaiotti E, Sigismondi C, Montoli S, Mangili G, Candiani M, Vigano R. Management of locally advanced cervical cancer in pregnancy: a case report. Tumori 2010;96:623-626. PMID: 20968145.


13. Zemlickis D, Klein J, Moselhy G, Koren G. Cisplatin protein binding in pregnancy and the neonatal period. Med Pediatr Oncol 1994;23:476-479. PMID: 7935173.


14. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177-183. PMID: 10329031.


15. Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol 2006;103:618-622. PMID: 16777200.


Fig.┬Ā1
(A) Speculum examination shows an approximately 5-cm-sized mass on the cervix. (B) Magnetic resonance imaging (midsagittal T2-weighted) in a pregnant patient at 25 weeks' gestation before neoadjuvant chemotherapy shows a 5.4├Ś5├Ś5-cm cervical tumor.
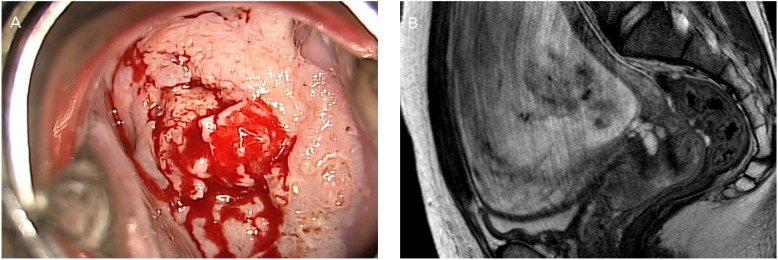
Fig.┬Ā2
(A) Magnetic resonance imaging (midsagittal T2-weighted) in a pregnant patient at 33 weeks' gestation after neoadjuvant chemotherapy shows a 2.5├Ś2├Ś2-cm cervical tumor. (B) Photograph of the macroscopic surgical specimen after cesarean delivery and radical hysterectomy. (C) Hematoxylin and eosin staining (├Ś200 objective lens) of squamous cell carcinoma, large cell, nonkeratinizing type.

Table┬Ā1
Neoadjuvant chemotherapy using paclitaxel plus cisplatin followed by cesarean delivery and radical surgery in cervical cancer during pregnancy
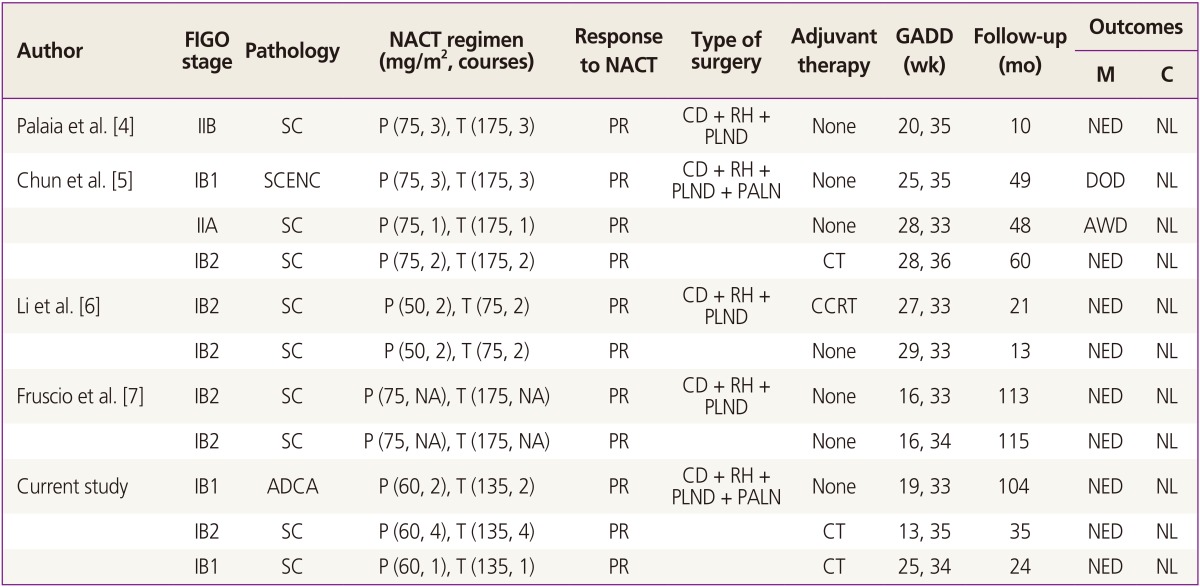
FIGO, International Federation of Gynecology and Obstetrics; NACT, neoadjuvant chemotherapy; GADD, gestational age at diagnosis and delivery; M, mother; C, child; SC, squamous cell carcinoma; P, cisplatin; T, paclitaxel; PR, partial response; CD, cesarean delivery; RH, radical hysterectomy; PLND, pelvic lymph node dissection; NED, no evidence of disease; NL, normal; SCENC, small cell neuroendocrine carcinoma; PALN, para-aortic lymph node dissection; AWD, alive with disease; CT, chemotherapy; CCRT, concurrent chemoradiation therapy; NA, not available; ADCA, adenocarcinoma.
-
METRICS

-
- 6 Crossref
- 2,795 View
- 31 Download
- Related articles in Obstet Gynecol Sci



















