Hyperplastic primary vitreous with hemorrhage manifested as a hyperechoic mass in the fetal orbit by prenatal ultrasound in a case of isolated unilateral microphthalmia
Article information
Abstract
Congenital microphthalmia is a rare anomaly of the fetal orbit resulting from developmental defects of the primary optic vesicle. Chromosomal anomalies, genetic defect, infection, and prenatal drug exposure are the most common causes. Congenital microphthalmia is usually associated with other abnormalities, and cases of isolated microphthalmia are rarely reported. Congenital microphthalmia can be diagnosed by prenatal ultrasound by measuring the axial diameter of the eye ball, but the accuracy depends on fetal position and associated anomalies. We report a case of an isolated unilateral microphthalmia which was not diagnosed by prenatal ultrasound, because the only abnormal prenatal ultrasound finding was a small hyperechoic mass lesion in the eye ball and the subsequent scan of the orbits was limited due to fetal prone position. The hyperechoic mass lesion in the eye ball was finally diagnosed as a persistent hyperplastic primary vitreous with hemorrhage by neonatal magnetic resonance image.
Introduction
Congenital microphthalmia is a rare anomaly of the fetal orbit resulting from developmental defects of the primary optic vesicle [1]. The incidence of microphthalmia is reported as 0.7 to 1.9 per 10,000 births [23]. Chromosomal anomalies, genetic defect, infection, and prenatal drug exposure are the most common underlying causes [14]. Congenital microphthalmia is usually associated with systemic abnormalities (50% to 90%) and various developmental ocular anomalies, including coloboma, lens and optic nerve defects [56], and therefore an isolated microphthalmia is a rare congenital disease.
With the recent advances in obstetric ultrasound, congenital microphthalmia can be diagnosed by prenatal ultrasound when the axial diameter of the eye ball is less than 2 standard deviation below the mean [7]. But the accuracy depends on fetal position and associated anomalies. When the fetus is in a prone position, accurate fetal eye examination is limited, especially if the eye abnormality is an isolated finding.
We report a case of an isolated unilateral microphthalmia, in which the only abnormal ultrasound finding during the second trimester ultrasound was a hyperechoic mass lesion at the posterior medial side of the lens. Microphthalmia of the case was diagnosed and confirmed by postnatal examination including physical examination, ultrasound and magnetic resonance image (MRI).
Case report
A 27-year-old, nulliparous woman was referred to our institution at 29 gestational weeks for the evaluation of incidental abnormal finding in the right eye detected by routine midtrimester screening ultrasound. The level II ultrasound in our hospital showed no structural anomaly including brain, heart and kidney except for a 6.2-mm-sized hyperechoic mass lesion in the right eye ball at posterior medial side of the lens adjacent to the hyaloid artery (Fig. 1A). The lens was normal and there was no specific finding of congenital cataract (Fig. 1B). The right and left orbital axial diameter was 13.2 and 13.5 mm, respectively, which were in the normal ranges (Fig. 1C). The couple was healthy without a history of genetic disorder. There was no history of medication use during pregnancy, exposure to X-rays, or maternal infection. Other prenatal examinations including viral screening test, the sequential test and 50 g oral glucose tolerance test were normal.

(A) A hyperechoic mass lesion (6.2 mm, arrow) in the right eye ball at posterior medial side of the lens adjacent to the hyaloid artery by ultrasound at 29 weeks of gestation. (B) A normal right lens with no specific finding of congenital cataract by ultrasound at 29 weeks of gestation. (C) The right and left orbital coronal diameter was 13.2 and 13.5 mm by ultrasound at 29 weeks of gestation. (D) A 6.6-mm-sized hyperechoic mass lesion in right eye ball by ultrasound at 31 weeks of gestation.
Subsequent ultrasound assessment was done at 31, 34, and 38 week of gestation. The size of the hyperechoic mass lesion in right eye ball was 6.6 mm at 31 week of gestation (Fig. 1D). The estimated fetal weight, amniotic fluid and other fetal structures were all normal. However, the subsequent scan of the orbits was limited due to fetal prone position, thus orbital diameters (ODs) were not measured correctly.
The women delivered a male neonate weighing 3.44 kg vaginally at 41 weeks of gestation. The newborn baby did not open his right eye spontaneously. The ophthalmologist examined the baby and right congenital microphthalmia was diagnosed with coloboma, small orbital fissure, iris atrophy and hyperpigmentation of the fundus. Other facial features were normal. Postnatal orbit MRI showed right microphthalmia including globular and dysmorphic right lens (Fig. 2A). A soft tissue lesion at posteromedial aspect of the right lens (Fig. 2B) was found in the orbit MRI which was the hyperechoic mass lesion in the right eye ball detected by prenatal ultrasound. This lesion was diagnosed as a persistent hyperplastic primary vitreous with hemorrhage (Fig. 2C). Right optic nerve was atrophic but brain parenchyma was normal (Fig. 2D). Echocardiogram and abdominal ultrasound including liver, gallbladder, pancreas, and kidney were performed for associated systemic abnormalities, but there was no significant associated abnormality. The chromosome analysis and genetic test were not performed. After full work up, the baby was sent home with a plan to have a prosthetics unit at a later time during childhood.
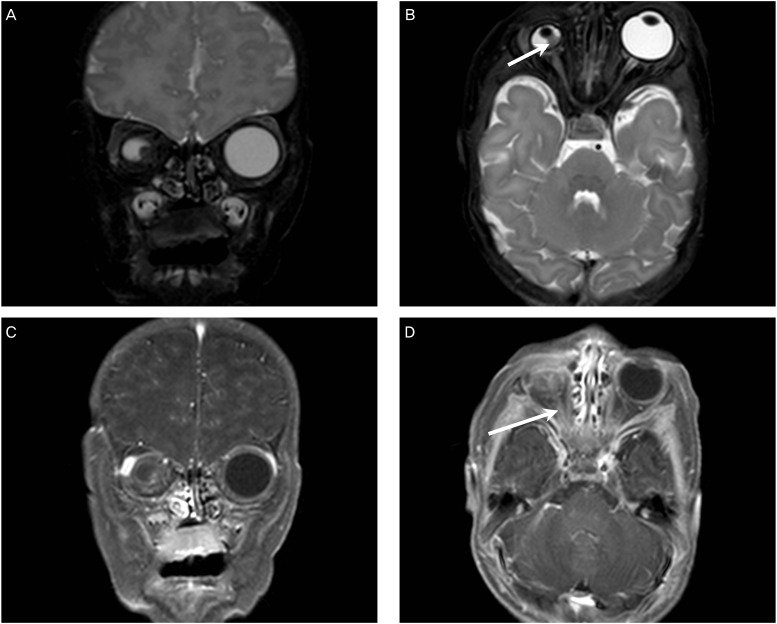
(A) Right microphthalmia by postnatal orbit magnetic resonance image (MRI), T2 weighted, coronal image. (B) A soft tissue lesion at posteromedial aspect of the right lens by postnatal orbit MRI, T2 weighted, axial image (arrow). (C) Right microphthalmia with persistent hyperplastic primary vitreous with hemorrhage by postnatal orbit MRI, T1 weighted, coronal image. (D) Right optic nerve atrophy (arrow) by postnatal orbit MRI, T1 weighted, axial image.
Discussion
Fetal eye can be detected by transvaginal ultrasound as early as 12 weeks of gestation [8]. Using the coronal or axial planes of the fetal head, assessment of fetal eyes can be done easily. Normal fetal eyes appear as round, symmetrical structures with hypoechoic inner contents, and normal lenses appear as a round ring with echogenic rim located in the anterior portion of the glove. During fetal ultrasound examination, fetal orbital biometry, including OD, interorbital distance and binocular distance, can be obtained in the axial plane. Precise measurement of the fetal orbital biometry is mandatory for diagnosing various congenital ocular anomalies such as hypotelorism, hypertelorism, anophthalmia and microphthalmia. A detailed examination of the fetal eye should also include examining the lens, pupil, palpebral fissure and intraorbital anatomy.
Fetal OD is calculated by measuring the maximal transverse diameter of the orbit in the axial plane. The mean OD is about 10 mm in 20 weeks of gestation and gradually increased up to 15 mm at term [9]. Microphthalmia is detected when the size of the eye is unusually small, as early as from the early second trimester [10]. The diagnosis of microphthalmia is made when the OD is less than 2 standard deviation below the mean or 5th percentile for gestational age [11]. Bilateral microphthalmia is more common and can be detected more easily. Bilateral microphthalmia is commonly associated with other systemic anomalies including holoprosencephaly, neural tube defects, vertebral and rib abnormalities, cleft palate, ectopic kidney, horseshoe kidney, and congenital heart defects. However, unilateral microphthalmia is usually isolated [2]. The fetus in our case had no other associated anomalies and the only abnormal finding was a hyperechoic mass lesion in the right eye ball at posterior medial side of the lens. A small difference in size existed between the right eye and left eye, but the difference was not diagnostic. And because of fetal prone position, we were not able to evaluate the fetal eyes properly during the subsequent ultrasound, and therefore the final diagnosis was made only after birth.
The early embryology of the eye includes several important events including optic vesicle formation, optic cup formation, and optic fissure closure, which are associated with various molecular signals, pathways, and genetic coordinators [12]. Congenital micropthalmia may result from any abnormal embryologic events such as incomplete closure of the embryonic fissure. Chromosomal anomalies and genetic mutations are the most common underlying causes [4]. SOX2 and OTX2 have been identified as a major mutated gene known to cause microphthalmia [1314]. Environmental factors including congenital infection, maternal vitamin A deficiency, hyperthermia, fever, X-rays exposure and drug exposure are other underlying causes. But, exact cause may not be identified in many cases.
When microphthalmia is diagnosed prenatally, a detailed evaluation for underlying cause and associated anomaly should be done. Familial history including parental eye examination and amniocentesis for fetal karyotyping and genetic analysis are recommended. Maternal serum test is needed to identify intrauterine infections, such as varicella, toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus [15]. A targeted ultrasound should be done to identify other combined anomaly.
After birth, microphthalmia is easily identified by inspection and palpation of the eye. When the final diagnosis is made, the baby should be referred for an ophthalmologist assessment. Evaluation of facial morphology including palate and ear, cardiac, musculoskeletal, urogenital, neurological system, esophagus for dysphagia and syndromes should be undertaken [14]. Chromosome analysis, genetic analysis and infection study, if not performed prenatally, should be done, especially when the microphthalmia is bilateral or associated with other anomalies. In our case, detailed ophthalmological and pediatric assessments were performed. However, because the neonate was found to have an isolated unilateral microphthalmia, genetic test including karyotyping was not performed.
The aim of management of microphthalmia is to maximize visual the potential and improve the cosmetic outcome. The management of microphthalmia includes socket expansion for preventing facial asymmetry, oculoplastic surgery, and prosthetics unit [1]. Microphthalmia may progress to glaucoma, which may result in pain and loss of vision. Other visual problems with severe microphthalmia include a reversal of sleep pattern.
In summary, microphthalmia is a rare prenatally diagnosable congenital anomaly. However, it was not correctly diagnosed by prenatal ultrasound in our case because the lesion was unilateral, the fetus had no associated anomaly, and the follow up ultrasound exams were limited due to fetal prone position. The only abnormal ultrasound finding during the second trimester ultrasound was a hyperechoic mass lesion at the posterior medial side of the lens, which was finally diagnosed as a persistent hyperplastic primary vitreous with hemorrhage by neonatal MRI. Microphthalmia may progress throughout gestation and early prenatal diagnosis may be impossible [10]. Therefore, a small abnormal ultrasound finding such as a hyperechoic mass in the eye ball should be observed carefully, and ensure a postnatal evaluation.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.