Intrapelvic dissemination of early low-grade endometrioid stromal sarcoma due to electronic morcellation
Article information
Abstract
Endometrioid stromal sarcoma is a rare malignancy that originates from mesenchymal cells. It is classified into low-grade endometrioid stromal sarcoma (LGESS) and high-grade endometrioid stromal sarcoma. Ultrasonographic findings of LGESS resemble those of submucosal myomas, leading to the possible preoperative misdiagnosis of LGESS as uterine leiomyoma. Electronic morcellation during laparoscopic surgery in women with LGESS can result in iatrogenic intraabdominal dissemination and a poorer prognosis. Here, we report a patient with LGESS who underwent a supracervical hysterectomy and electronic morcellation for a presumed myoma in another hospital. Disseminated metastatic lesions of LGESS in the posterior cul-de-sac and rectal serosal surface were absent on primary surgery, but found during reexploration. In conclusion, when LGESS is found incidentally following previous morcellation during laparoscopic surgery for presumed benign uterine disease, we highly recommend surgical reexploration, even when there is no evidence of a metastatic lesion in imaging studies.
Introduction
Endometrioid stromal sarcoma, a rare malignancy that originates from mesenchymal cells, accounts for 0.2% of all uterine malignancies. Endometrioid stromal sarcoma is classified into low-grade endometrioid stromal sarcoma (LGESS) and high-grade endometrioid stromal sarcoma [1]. Ultrasonographic findings of LGESS resemble those of submucosal myomas, leading to the possible preoperative misdiagnosis of LGESS as uterine leiomyoma [2]. Therefore, laparoscopic myomectomy and electronic morcellation may be carried out in women with LGESS for presumed myoma, resulting in iatrogenic intraabdominal dissemination and a poorer prognosis [3].
Recently the Food and Drug Administration (FDA) reported the risk of using an electronic morcellator, due to the fact that the prevalence of unsuspected uterine sarcoma and leiomyosarcoma during a myomectomy for a presumed myoma was 0.28% and 0.2%, respectively [456]. The FDA also warned that laparoscopic power morcellation poses a risk of spreading unsuspected cancerous tissue beyond the uterus, notably uterine sarcomas, when used for a hysterectomy or a myomectomy in women with uterine fibroids [5].
There exists limited literature pertaining to the management of patients with inadvertently morcellated uterine sarcomas. The authors of 2 small studies recommend immediate reexploration in the case of incidentally uncovering uterine malignancy after morcellation or supracervical hysterectomy for presumed benign uterine disease [78].
Here, we report a patient with LGESS who underwent a supracervical hysterectomy and electronic morcellation for a presumed myoma in another hospital. Disseminated metastatic lesions of LGESS in the posterior cul-de-sac and rectal serosal surface were found during reexploration, but were absent at the time of primary surgery.
Case report
A 46-year-old woman (gravida 2, para 2) was transferred to our department due to a postoperative diagnosis of LGESS, that was found incidentally after a supracervical hysterectomy and morcellation for a presumed uterine myoma. She presented preoperatively with abnormal vaginal bleeding, and reported no previous medical disease. We performed abdominopelvic computed tomography (CT) in conjunction with positron emission (PET)-CT. Although CT and PET-CT showed no evidence of a metastatic tumor, we still recommended surgical reexploration due to the fact that electronic morcellation during surgery may have caused abdominopelvic dissemination of tumor cells.
The patient underwent reexploratory surgery approximately 3 weeks after the initial surgery. During the reexploratory surgery, numerous small metastatic lesions were found in the cul-de-sac and rectal serosal surface, however there was no other visible evidence of metastatic lesions in the abdomen. Due to the fact that the metastatic lesions were diffusely infiltrative on the rectal surface, a low anterior resection was required in order to completely remove the metastatic lesions. The entirety of the visible metastatic lesions could be completely removed via a low anterior resection, bilateral salpingo-oopherectomy, and removal of the remaining cervical stump and cul-de-sac. Additionally, an omentectomy and an appendectomy were carried out. Due to the fact that there were no enlarged palpable pelvic or paraaortic lymph nodes suggestive of metastasis, a pelvic and paraaortic lymph node dissection was not performed.
The Postoperative course was uneventful. We reviewed the video recording of the initial surgery at the other hospital, and found no abnormal lesions on the rectal surface (Fig. 1). Given the findings on initial and reexploratory surgery, the rectal metastasis was likely due to dissemination from the electronic morcellation.
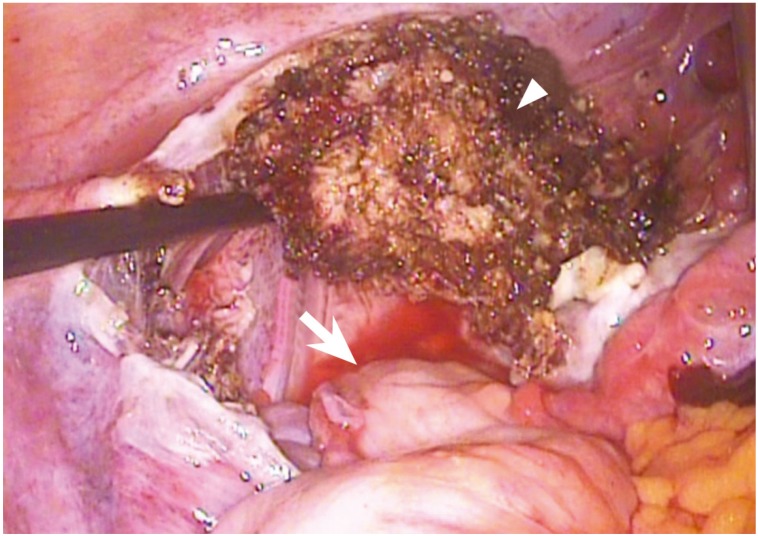
The initial surgical findings of the rectal surface. The figure shows the abdominopelvic cavity after removal of the uterus by supracervical hysterectomy. There is no sign of metastasis in the rectal surface (arrow, rectal surface; arrowhead, remnant electrocauterized cervix).
Pathological examination of the tissue confirmed a metastatic LGESS involving the resected rectal serosal surface (Fig. 2). There were no other metastatic lesions. The LGESS was positive for the estrogen receptor, progesterone receptor, smooth muscle actin, CD10, P53, and Ki-67. The patient was diagnosed with stage IIB LGESS. Progesterone medication (medroxyprogesterone acetate 10 mg/day) was administered as a postoperative adjuvant treatment. There was no evidence of disease recurrence during the 12-month follow-up after reexploratory surgery.

The resected rectum. (A) The gross finding of the resected rectum. The arrow indicates metastatic lesions of the rectal surface, which has an irregular surface contour. The arrowhead indicates the adjacent normal rectal serosa. (B) Low-power field microscopy shows a tumor (T) of the rectal surface, muscle layer (Ms) and mucosal layer (Mu) of the rectum (H&E stain, ×20). (C) H&E stain, ×400. (D) Immunohistochemical staining shows a positive indication of CD10 (×200).
Discussion
LGESS is characterized by high sensitivity to progesterone therapy and an indolent clinical course. The five year survival rate of stage I is 98% and the 10-year survival rate is 89%. In advanced disease, the overall survival rate of stage III/IV is much lower, at 66%, and the overall recurrence rate of this stage is 76%, being the most common recurrent lesion found in the pelvis and abdomen. Recurrence can occur 10 to 20 years after the initial diagnosis [2]. LGESS usually occurs in younger women aged 40 to 55 years old. Abnormal uterine bleeding and uterine enlargement is the most common symptom, whereas 25% of cases are asymptomatic.
Recently, the FDA warned that laparoscopic morcellation poses a risk of spreading unsuspected uterine sarcomas, when used for a hysterectomy or myomectomy in women with presumed uterine myomas [5]. However, the most prevalent opposing view [9] is derived from the low incidence (approximately 0.28%) of unsuspected uterine sarcoma during myomectomy for presumed myoma [456]. Given the extremely low prevalence of sarcoma in a series of laparoscopic surgeries performed for presumed myomas, and the lack of a specific diagnostic method to differentiate between the two, gynecological surgeons are faced with the dilemma of whether all surgeries performed for presumed myomas should be carried out via a non-laparoscopic surgical route [9].
The majority of patients with LGESS are diagnosed at an early stage of the disease that is confined to the uterus, and have a good prognosis [10]. However, the usage of electronic morcellation during tumor removal in early-staged LGESS patients leads to an intrapelvic and intraabdominal dissemination, and as a result, a poorer prognosis [3]. Such a clinical problem is caused by the lack of a specific diagnostic method to differentiate between uterine sarcoma and myoma [2311].
There are no appropriate guidelines for the treatment of incidental uterine malignancy found after morcellation during laparoscopic surgery for presumed benign uterine disease. Two previous studies included only a small number of patients with LGESS and/or leiomyosarcoma, and the authors recommended immediate reexploration in patients with inadvertently morcellated uterine sarcomas for accurate staging, prognostic information, and suitable postoperative treatment [78]. A previously reported case was similarly postoperatively diagnosed as LGESS, subsequent to a laparoscopic supracervical hysterectomy and uterine morcellation due to a presumed myoma. The patient underwent a laparotomy 2 months after the initial surgery, and gross metastatic lesions were found in both ovaries and fallopian tubes [12]. Based on these previous reports, we are in agreement with both Einstein et al. [7] and Oduyebo et al. [8] with respect to the need for immediate reexploratory surgery. Furthermore, our case demonstrates that disseminated metastatic lesions may not be detected in imaging studies, including CT and PET-CT. Therefore, we recommend surgical reexploration despite the absence of metastatic lesions in imaging studies.
Although there are no appropriate guidelines for the treatment of incidental uterine malignancy found after morcellation during laparoscopic surgery, we believe that the therapeutic approach should be based on the guidelines present for primary therapy of LGESS, and not for that of recurrent disease. The intrapelvic and intraabdominal dissemination caused by the usage of electronic morcellation need to be removed [78], due to the fact that the disseminated lesions lead to a poorer prognosis [3]. Furthermore, previous studies found no clear evidence that adjuvant chemotherapy, radiotherapy, and hormone therapy decrease the recurrence of LGESS or improve patient survival [13], therefore we do not believe that such adjuvant therapy alone can replace the surgical removal of disseminated lesions. Based on such a therapeutic concept, the treatment preference in our department is surgical removal of all the disseminated lesions, followed by postoperative adjuvant progesterone therapy.
Alternative methods to electronic morcellation are urgently needed. Proposed alternative methods include laparoscopic assisted minilaparotomy, tissue removal by vaginal incision, and manual morcellation within an endobag [614].
In conclusion, we recommend surgical reexploration for incidental LGESS after morcellation during laparoscopic surgery for presumed benign uterine disease, even when there is no evidence of metastatic lesions in imaging studies.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.