Adult granulosa cell tumor presenting with massive ascites, elevated CA-125 level, and low 18F-fluorodeoxyglucose uptake on positron emission tomography/computed tomography
Article information
Abstract
Adult granulosa cell tumors (AGCTs) presenting with massive ascites and elevated serum CA-125 levels have rarely been described in the literature. An ovarian mass, massive ascites, and elevated serum CA-125 levels in postmenopausal women generally suggest a malignant ovarian tumor, particularly advanced epithelial ovarian cancer. AGCT has low 18F-fluorodeoxyglucose uptake on positron emission tomography/computed tomography due to its low metabolic activity. In the present report, we describe a case of an AGCT with massive ascites, elevated serum CA-125 level, and low 18F-fluorodeoxyglucose uptake on positron emission tomography/computed tomography.
Introduction
Granulosa cell tumors comprise only 5% of all ovarian malignancies and account for approximately 70% of malignant sex cord-stromal tumors [12]. The majority of patients present with one or a combination of the following clinical symptoms: abnormal vaginal bleeding, abdominal distension, and abdominal pain [3]. Adult granulosa cell tumors (AGCTs) are low-grade malignancies with a propensity to remain localized and demonstrate indolent growth. Ninety percent are stage I at diagnosis [4]. Granulosa cell tumors presenting with massive ascites have rarely been described in the literature [5678]. To our knowledge, only a few cases of AGCT with massive ascites and elevated serum CA-125 levels have been reported [68]. An ovarian mass, massive ascites, and elevated serum CA-125 levels in postmenopausal women generally suggest a malignant ovarian tumor, especially advanced epithelial ovarian cancer. In the differential diagnosis of ovarian cancer from benign tumors, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) demonstrates high accuracy [9]. The maximum standardized uptake value (SUVmax) is a noninvasive method to detect biochemical and metastatic changes in cancer tissue; malignant ovarian tumors have a SUVmax >2.9 [10]. Moreover, 18F-FDG PET/CT had a high diagnostic value in differentiating between malignant and benign tumors. The mean SUVmax of malignant ovarian tumor was 9.32±4.58 [11]. However, the diagnostic role of 18F-FDG PET/CT in AGCT is not well described due to the low level of proliferation and metabolic activity exhibited by AGCTs. Herein, we report a case of an AGCT presenting with massive ascites, elevated serum CA-125 level, and low 18F-FDG uptake on PET/CT.
Case report
A 55-year-old postmenopausal woman was admitted to our hospital with a 1-month history of abdominal fullness and dyspnea. She had no specific medical or surgical history. An ultrasound revealed massive ascites and a heterogeneous mass measuring 15×14×13 cm3 in the lower abdomen. Computed tomography (CT) also revealed a well-defined solid pelvic mass including a cystic component measuring 15×14×13 cm3 arising from the left adnexa (Fig. 1A). 18F-FDG PET/CT showed low peripheral 18F-FDG uptake (SUVmax 2.1) (Fig. 1B). CT and 18F-FDG PET/CT showed no evidence of metastatic disease involving the lymph nodes or abdominal organs. The serum CA-125 concentration was 220.5 U/mL (upper limit, 35 U/mL). The serum levels of carcinoembryonic antigen and CA-19-9 were normal. An explorative laparotomy was performed based on the suspicion of ovarian cancer. The left ovary was replaced by a mass measuring 14×13×12 cm3, and 5,000 mL serous ascites fluid was aspirated from the abdomen. The liver, spleen, bilateral paracolic gutters, bowel, and the space beneath the diaphragm were all free of tumor. A total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and pelvic and paraaortic lymphadenectomy were performed. Intraoperative cytology was also performed. Frozen section from tumor specimen showed sex cord stromal tumor which was suggestive of granulosa cell tumor.

(A) Computed tomography showing a 15×14×13 cm3 well-defined solid pelvic mass including a cystic component (arrow) with massive ascites. (B) 18F-fluorodeoxyglucose positron emission tomography/computed tomography showing low peripheral 18F-fluorodeoxyglucose uptake (maximum standardized uptake value 2.1, arrow).
The final histopathology results revealed an AGCT. Grossly, the tumor had a yellow to tan color, with a uniloculated cyst containing solid components with mural thickening or trabeculated intraluminal masses (Fig. 2A). Microscopically, the tumor showed mixed growth patterns, including diffuse (70%) and anastomosing trabecular (20%) patterns. It also displayed microfollicular (5%) and gyriform (5%) features (Fig. 2B), although these were less common. Tumor cells showed relatively uniform, oval, angular, and often grooved nuclei with scant cytoplasm (Fig. 2C). Some foci showing a microfollicular pattern with eosinophilic and hyalinized components were identified as Call-Exner bodies. The mitotic activity was 1 to 2 mitotic figures/10 high power fields. Tumor cell necrosis was not observed. The ipsilateral peritubal soft tissue adherent to the ovary was infiltrated by the tumor cells. However, the contralateral ovary and fallopian tube, uterus and omentum, as well as multiple pelvic soft tissues and 35 pelvic lymph nodes all tested negative for malignancy. Additionally, intraoperative cytological examination of the ascites showed no evidence of malignant cells. Immunohistochemical results indicated that the tumor was diffusely positive for calretinin (Fig. 2D), focally and weakly positive for alpha-inhibin (Fig. 2E), and negative for cytokeratin 7 (Fig. 2F). This immunonegativity for cytokeratin 7 can exclude the possibility of undifferentiated primary or metastatic carcinoma.
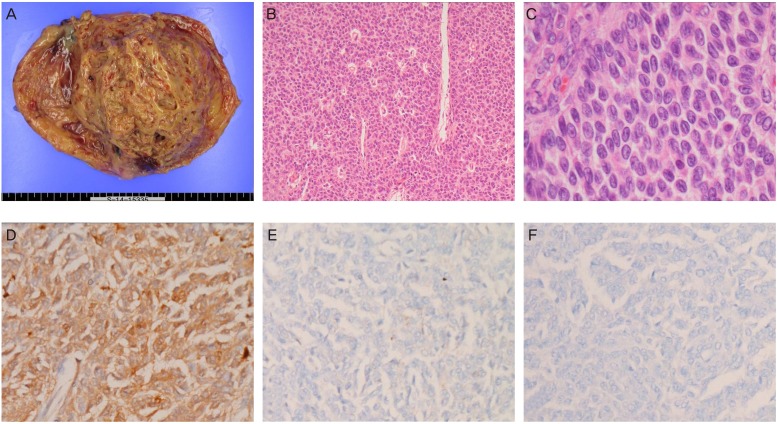
(A) Gross findings of the left ovarian tumor. (B, C) Histopathological features of the tumor cells (hematoxylin and eosin stain; B, ×100; C, ×400). (D-F) Immunohistochemical staining of the tumor cells (D, calretinin; E, alpha-inhibin; F, cytokeratin 7; D-F, ×200).
The final FIGO (International Federation of Gynecologists and Obstetricians) stage was IA. No adjuvant therapy was added. The patient had an uneventful postoperative course. The serum CA-125 level had normalized 3 months after surgery. In addition, the serum level of inhibin was normal (0.9 pg/mL).
Discussion
AGCT is a rare ovarian tumor, and it is important that all gynecologists, especially gynecologic oncologists, are aware of its clinical presentation and appropriate treatment. A rare association has been shown between Meigs syndrome and granulosa cell tumor; Meigs syndrome is defined as the triad of ovarian tumor with ascites and pleural effusion that disappears after tumor resection [12]. Histologically, an ovarian tumor may be a fibroma, thecoma, cystadenoma, or granulosa cell tumor. Fluid accumulation may be due to vasoactive substances, such as vascular endothelial growth factor, that increase capillary permeability. These substances are released by the tumor or the surrounding vasculature due to tumor pressure, leading to lymphatic congestion in the peritoneum [7].
Although CA-125 is a tumor marker for malignant epithelial ovarian cancer, it can also be elevated in benign conditions due to peritoneal and pleural irritation [13]. A previous review article suggested that elevation of serum CA-125 antigen level in patients with Meigs syndrome is caused by mesothelial expression of CA-125 rather than ovarian tumor cell expression [12]. Due to the rarity of AGCT, massive ascites with an elevated serum CA-125 level caused by an AGCT may be misdiagnosed as advanced epithelial ovarian cancer or primary peritoneal carcinoma.
In our case, results of the cytologic examination of the ascites were negative for malignancy. AGCT tends to present with stage I disease. An association with ascites and pleural effusion is found in approximately 1% to 2% of cases of AGCT; exfoliated tumor cells are rarely found in the ascitic fluid [14]. Previous case reports have indicated that preoperative cytologic examination of ascites and pleural effusion may help in the diagnosis of AGCT [612]. The differential diagnosis of AGCT on cytology includes solid ovarian tumors such as malignant germ cell tumors, adenocarcinomas and poorly differentiated Sertoli-Leydig cell tumors [6]. In the differential diagnosis of small monomorphic tumor cells in ascites or pleural effusion, coffee bean-shaped nuclear grooves and cell clusters forming Call-Exner bodies are diagnostic clues for AGCT [15]. Immunohistochemical examination results positive for inhibin and Mic-2 may be helpful in the diagnosis of AGCT, whereas germ cell tumors will show positivity for alpha feto-protein or human chorionic gonadotrophin [6].
Although the usefulness of 18F-FDG PET/CT in the staging and follow-up of the majority of ovarian cancer cases has been demonstrated, AGCT is known to produce false-negative results on 18F-FDG PET/CT due to low 18F-FDG activity. When clinical factors indicative of ovarian cancer, such as massive ascites and high serum CA-125 levels, are present with low 18F-FDG uptake on PET/CT, a diagnosis of AGCT with massive ascites should be considered.
In conclusion, an adnexal mass with massive ascites and an elevated serum CA-125 level suggests a malignant ovarian tumor, particularly advanced epithelial ovarian cancer. However, clinicians should be aware that an adnexal mass with massive ascites, elevated serum CA-125 level, and low 18F-FDG uptake on PET/CT may be AGCT with massive ascites.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.