|
 |
- Search
| Obstet Gynecol Sci > Volume 56(4); 2013 > Article |
Abstract
Pulmonary embolism (PE) and deep vein thrombosis (DVT) is a serious medical problem causing considerable morbidity and mortality. Risk factors of thrombosis are surgery, trauma, pregnancy, tumor, oral contraceptive as well as genetic risk factors though genetic risk factors were found in about 5% to 10% of cases. Yasmin, a combined oral contraceptive containing ethinylestradiol 30 ┬Ąg and drospirenon 3 mg was launched in the United Kingdom in 2002. We had experienced a patient, 24-year-old young woman with left inguinal pain on ambulation, and edema of left leg four months after taking Yasmin. We performed chest pulmonary angiography computed tomography (CT) and lower extremity venogram CT. She was diagnosed to PE in both lower lobes and DVT below the level of left external iliac vein, and treated by low molecular weight heparin and warfarin. We report this case with brief review of literatures.
Combined oral contraceptives (COCs) are a common method of contraception, but they carry a risk of venous and arterial thrombosis. The association between estrogen-containing oral contraceptives (OCs) and venous thromboembolism (VTE) is well established [1]. Until 1995, it was generally thought that the progestogen component of COCs did not contribute to the risk of VTE. However, a number of studies published in late 1995 and early 1996 reported an increased risk of VTE for users of the so-called third-generation COCs containing the progestogens, desogestrel or gestodene, compared with second-generation COCs, containing levonorgestrel [2]. These findings were unexpected and led to debate over possible bias and confounding. New studies, re-analyses of the original studies and a meta-analysis have since been published [3]. VTE is rare event in young women and the risk associated with a new COC. But, many studies indicate that the well-known risk of VTE related to the use of COC needs to be considered as a cause of pulmonary embolism (PE) even among very young females [4]. So, prevention of these life-threatening conditions in patients without a family history of VTE consists of timely examination of their thrombotic profile and selection of appropriate medication.
We have experienced a patient, 24-year-old young woman with PE and deep vein thrombosis (DVT) without inherited risk factors four months after taking Yasmin. We report this case with brief review of literatures.
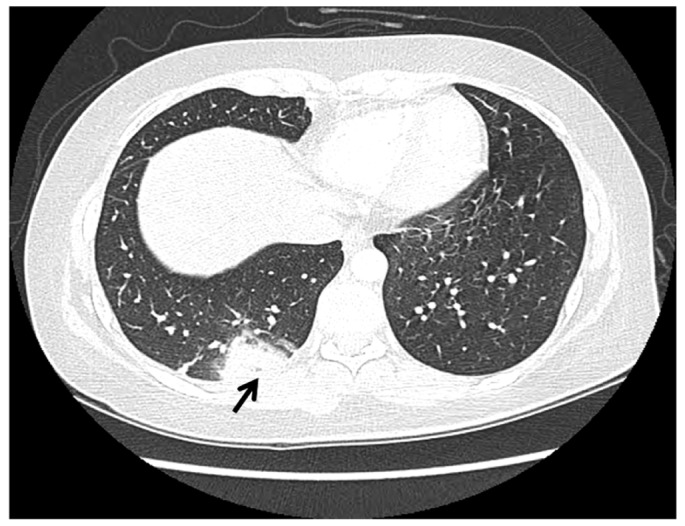
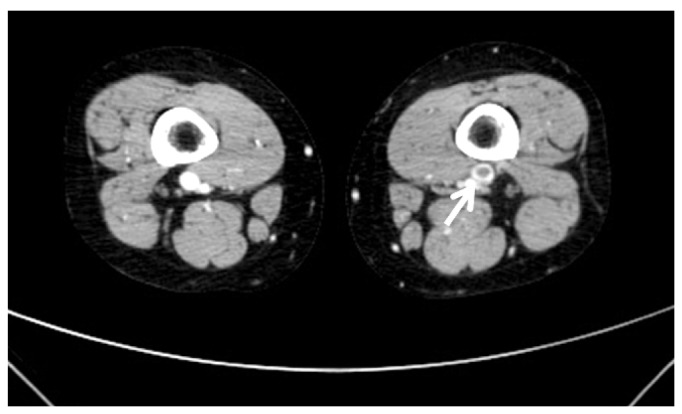
A 24-year-old woman (gravida 0) visited our clinic due to left inguinal pain on every step and edema of left leg four months after taking Yasmin (Bayer HealthCare, New York, NY, USA). This pain and edema were started a week ago and aggravated three days ago. Her weight was 69 kg, and height 170 cm. She was a non-smoker. Her job was a company worker. The patient and family members had not suffered prior VTE. She received pelviscopic ovarian cystectomy of 5 cm sized left ovarian endometrioma at another clinic a year ago. Two weeks after operation, she was injected gonadotropin releasing hormone (GnRH) per four weeks for six months continuously. A month after sixth GnRH injection, she had started taking Yasmin 21-tablet continuously without seven-day discontinuation period for four months to prevent recurrence of endometriosis. During taking the fifth pack of Yasmin, she felt left inguinal pain on ambulation, and edema of left leg. So, she admitted our clinic. Her vital signs were as stable. Body temperature 120/80 mm Hg, pulse rate 72/min, respiratory rate 20/min, body temperature 36.5Ōäā. She had no chest discomfort, breathing difficulties and skin color change of left leg. The circumference of her left thigh was proximal 64 cm, distal 48 cm, and right proximal 57 cm, distal 43 cm, respectively. Her left radial artery and dorsalis pedis artery were palpable well. Laboratory findings were below. Complete blood count, chemistry profile, and urine analysis were all normal range. Prothrombin time 11.6 sec (normal, 9.8 to 12.4 sec), partial thromboplastin time 96.6% (normal, 80% to 135%). Anti-cardiolipin antibody IgG/IgM, Lupus anticoagulant, Factor V Leiden (FV, Q506) were all negative. Protein C activity was 109% (normal, 70% to 130%), and protein S activity 99% (normal, 65% to 140%). D-dimer was elevated to 2.68 ┬Ąg/mL (normal, 0 to 0.5 ┬Ąg/mL). Chest posterial-anterial and electrocardiogram were normal findings. Chest pulmonary angiography computed tomography (CT) (contrast enhanced) showed pulmonary thromboembolism in BLLs' basal pulmonary arteries and 32-mm sized consolidation in right lower lobe posterior segment as considering pulmonary infarction (Fig. 1). Lower extremity venogram CT (contrast enhanced) showed thrombosis in inferior vena cava (IVC) and diffuse DVT below the level of left external iliac vein (Fig. 2). We injected low molecular weight heparin (LMWH) 60 mg per 12 hours subcutaneously for seven days and overlapped heparin with 4 mg warfarin five days. After five days of combined therapy, we changed to 4 mg warfarin per oral. We determined the proper dose of warfarin by a blood test reported as the international normalized ratio. She discharged with warfarin. She had taken warfarin for six months thereafter. She had normal menstrual cycles and no recurrence of PE and DVT during 12-month follow-up.
VTE poses a public health threat with an estimated incidence in the United States of 250,000 to 2 million cases per year. Predisposition to VTE arises from acquired conditions, inherited disorders, or both. Acquired risk factors include obesity, cigarette smoking, hypertension, immobilization, surgery, trauma, oral contraceptives, pregnancy, and hormone replacement therapy. Chronic medical illnesses such as congestive heart failure, chronic obstructive pulmonary disease, and cancer also predispose to PE. Heredity plays an important role in a patient's susceptibility to PE. So, we do genetic tests such as factor V Leiden and the prothrombin gene mutation that can identify those who are predisposed. The presence of these risk factors is sometimes called a prothrombotic or thrombophilic state. DVT most often originates in the calf, with a persistent cramping or "charley horse" that intensifies over several days. Leg swelling and discoloration may accompany the increase in discomfort. Upper-extremity DVT may cause otherwise unexplained upper arm or neck swelling. The most frequently used diagnostic imaging test is the noninvasive venous ultrasound examination. To establish the diagnosis of PE, the most frequently used noninvasive imaging test is the rapid-speed chest CT scan. If D-dimer is normal, PE is extremely unlikely. Chest CT scan, which directly images blood clots causing blockages in the pulmonary arteries. Lung scan, which indirectly identifies areas of decreased blood flow in the lung tissue as a consequence of PE.
Anticoagulation begins with a combination of heparin and warfarin. The traditional unfractionated form ordinarily requires intravenous administration. More recently, LMWHs have begun replacing unfractionated heparin. In patients who cannot tolerate anticoagulation or those for whom anticoagulation fails, a permanent metal filter is inserted into the IVC, the largest vein below the heart, to prevent large blood clots from reaching the pulmonary arteries and causing PE. Unfortunately, the filter devices do not halt the clotting process. Their presence predisposes to future venous clots on or below the filter. The most controversial area in VTE therapy is the optimal duration of warfarin anticoagulation. The current recommendation is usually at least 6 months of anticoagulation, but it can sometimes be longer, according to individual patient circumstances [5].
Genetic risk factors were found in about 5% to 10% of cases. Activated protein C resistance caused by factor V Leiden mutation is the most common inherited cause of an underlying predisposition to PE and DVT. Factor V Leiden mutation is an important risk factor for PE or DVT during pregnancy, after pregnancy, or during oral contraceptive use [6]. The phenotypic expression of resistance to activated protein C is characterized by a poor response to the anticoagulant activity of activated protein C, a key enzyme in the down-regulation of blood coagulation, which causes a disposition for a hypercoagulable state. At least 90% of the cases with resistance to activated protein C are explained by a point mutation in the gene for coagulation factor V, resulting in replacement of an Arg to Gln at position 506 (factor V, Q506, often denoted factor V Leiden), one of the three activated protein C cleavage sites in activated factor V. Thrombotic events are often triggered through the presence of a combination of inherited and circumstantial risk factors. The high prevalence of activated protein C resistance raises the issue whether it would be cost-beneficial to screen for this trait in connection with surgery, pregnancy, and OCs [7].
The use of drospirenone-containing OCs was associated with an increased risk of DVT and PE, but not transient ischemic attack or cerebrovascular attack, relative to second- and third-generation COCs [8]. The risk of non-fatal VTE among users of OCs containing drospirenone seems to be around twice that of users of OCs containing levonorgestrel [9,10].
The risk of VTE in current users of COCs decreases with duration of use and decreasing estrogen dose. For the same dose of estrogen and the same length of use, OCs with desogestrel, gestodene, or drospirenone were associated with a significantly higher risk of VTE than COCs with levonorgestrel. Progestogen only pills and hormone releasing intrauterine devices were not associated with any increased risk of VTE [11]. The American College of Obstetricians and Gynecologists Committee said that when prescribing any oral contraceptive, clinicians should consider a woman's risk factors for VTE and decisions regarding choice of oral contraceptive should be left to their patients, taking into account the possible minimally increased risk of VTE, patient preference, and available alternatives [12].
Several specific potential risk factors for a fatal outcome from a COC-induced PE were identified. Recognition of these in combination with a high suspicion of VTE in COC users may reduce the risk of a fatal outcome. We have experienced a patient, 24-year-old young woman with PE and DVT without inherited risk factors four months after taking Yasmin. So, we report this rare case with brief review of literatures.
References
1. Vandenbroucke JP, Rosing J, Bloemenkamp KW, Middeldorp S, Helmerhorst FM, Bouma BN, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med 2001;344:1527-1535. PMID: 11357157.


2. Pearce HM, Layton D, Wilton LV, Shakir SA. Deep vein thrombosis and pulmonary embolism reported in the Prescription Event Monitoring Study of Yasmin. Br J Clin Pharmacol 2005;60:98-102. PMID: 15963100.



3. Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ 2001;323:131-134. PMID: 11463678.



4. Chroustova D, Kubinyi J, Jansa P, Veprekova L, Trnka J. V/P scan in diagnosis and follow-up of pulmonary embolism in 15-25-year-old females in relation to hormonal contraception use. Nucl Med Rev Cent East Eur 2011;14:63-67. PMID: 22219145.


5. Goldhaber SZ, Morrison RB. Cardiology patient pages. Pulmonary embolism and deep vein thrombosis. Circulation 2002;106:1436-1438. PMID: 12234943.


6. Hirsch DR, Mikkola KM, Marks PW, Fox EA, Dorfman DM, Ewenstein BM, et al. Pulmonary embolism and deep venous thrombosis during pregnancy or oral contraceptive use: prevalence of factor V Leiden. Am Heart J 1996;131:1145-1148. PMID: 8644593.


7. Rosen SB, Sturk A. Activated protein C resistance--a major risk factor for thrombosis. Eur J Clin Chem Clin Biochem 1997;35:501-516. PMID: 9263726.

8. Gronich N, Lavi I, Rennert G. Higher risk of venous thrombosis associated with drospirenone-containing oral contraceptives: a population-based cohort study. CMAJ 2011;183:E1319-E1325. PMID: 22065352.



9. Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011;342:d2151PMID: 21511805.



10. Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011;342:d2139PMID: 21511804.



11. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ 2009;339:b2890PMID: 19679613.



12. Committee on Gynecologic Practice. ACOG Committee Opinion Number 540: risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol 2012;120:1239-1242. PMID: 23090561.


-
METRICS

-
- 4 Crossref
- 2,889 View
- 27 Download
- Related articles in Obstet Gynecol Sci