Differences in clinical presentation and pregnancy outcomes in antepartum preeclampsia and new-onset postpartum preeclampsia: Are these the same disorder?
Article information
Abstract
Objective
New-onset postpartum preeclampsia is a poorly defined condition that accounts for a significant percentage of eclampsia cases. It is unclear whether new-onset postpartum preeclampsia is a different disorder from or belongs to the same spectrum of classic antepartum preeclampsia. The objective of this study was to compare the clinical presentation and pregnancy outcomes of antepartum preeclampsia and new-onset postpartum preeclampsia.
Methods
A retrospective study including 92 patients with antepartum preeclampsia and 92 patients with new-onset postpartum preeclampsia was performed. Clinical presentation and pregnancy outcomes were compared. Chi-square test was used to analyze categorical variables, and independent t-test and Mann-Whitney U-test for numerical variables. P-values of <0.05 were used to indicate statistical signifi cance.
Results
Patients with antepartum preeclampsia and new-onset postpartum preeclampsia differ significantly in profile, symptoms at presentation, laboratory markers and pregnancy outcomes.
Conclusion
New-onset postpartum preeclampsia has a distinct patient profile and clinical presentation than antepartum preeclampsia, suggesting they may represent different disorders. Characterization of a patient profile with increased risk of developing this condition will help clinicians to identify patients at risk and provide early and targeted interventions to decrease the morbidity associated with this condition.
Introduction
Preeclampsia is one of the leading causes of maternal morbidity and mortality in the world [12]. Most of the published literature has focused on its appearance during the antepartum period [3], which has translated into a decrease in the incidence of intrapartum eclampsia [45].
Conversely, preeclampsia in the postpartum period (i.e., new-onset postpartum preeclampsia) is a less-studied condition despite the fact that the reported prevalence of denovo postpartum hypertension or preeclampsia ranges from 0.3% to 27.5% [6] and that 0.3% of all postpartum visits to emergency departments are secondary to hypertension and preeclampsia [7]. As a matter of fact, approximately 50% of the cases of eclampsia develop after delivery [8], with roughly 26% of seizures developing more than 48 hours after birth [4], accounting for almost 15% of maternal deaths in the United States [4]. Despite the high incidence of eclampsia, pulmonary edema, stroke and thromboembolism [9], less attention has been given to postpartum preeclampsia [10] as there is paucity of data regarding incidence, risk factors, optimal treatment, and outcomes of hypertensive disorders diagnosed in the postpartum period [10].
The challenge clinicians encounter with new-onset postpartum preeclampsia is likely related to the inability to properly identify which patients are at risk of developing this condition, which usually occurs unexpectedly following an uneventful birth. Since the treatment of preeclampsia is delivery, the development of new-onset postpartum preeclampsia may suggest a different underlying pathophysiologic mechanism for this condition. The extent to which development or progression of postpartum preeclampsia is influenced by either the patient's demographic or antecedent obstetric characteristics is also unclear [11]. It is thus imperative that researchers work pon identifying women at increased risk of developing this disorder. By characterizing a profile of at-risk women, we would be able to provide early interventions, patient education, close monitoring and follow-up during the post-partum period resulting in decreased morbidity and mortality associated with this condition. Therefore, the purpose of this study was to investigate whether differences exist between antepartum and postpartum preeclampsia; and, in that case to characterize a profile of patients at increased risk of developing new-onset postpartum preeclampsia.
Materials and methods
This was a secondary analysis of de-identified records from patients with the diagnosis of preeclampsia that were admitted to Hutzel Women's Hospital in Detroit, MI, USA for management and subsequent delivery and who were followed as outpatients or readmitted during the postpartum period at our institution. The methodology and selection criteria of cases and controls identified from the medical records by using international classification of diseases 9th revision codes after institutional review board approval by the Wayne State University/Detroit Medical Center have been previously described [12].
The design of the current study was retrospective, non-experimental comparative. For purposes of the current study, cases with the diagnosis of preeclampsia according to the former standard criteria [13] were selected for analysis. Patient were subsequently categorized in two groups: antepartum preeclampsia (defined as preeclampsia diagnosed before delivery with no history of postpartum preeclampsia within 6 weeks postpartum); and new-onset delayed postpartum preeclampsia (defined as patients with diagnosis of postpartum preeclampsia 48 hours after delivery with no history of hypertension related disorders during pregnancy and/or within 48 hours of delivery). The exclusion criteria were any history of hypertensive-related disorders, as well as inadequate data.
Information considered for the current analysis were: demographics (maternal age, gravidity, parity, maternal race, prepregnancy body mass index [BMI], type of insurance, social history, and gestational age at delivery); medical history (diabetes, platelet disorder, and renal disease); clinical presentation (abdominal pain, headache, nausea/vomiting, shortness of breath, visual symptoms, seizures, blood pressure, use of magnesium, growth restriction, and abruption), laboratory data (biochemical and hematologic markers routinely ordered at our institution on these specific cases, including platelet count and mean platelet volume (MPV) at first prenatal visit as a routine, and also at hospital admission for preeclampsia), and neonatal outcomes (preterm delivery, newborn weight, newborn gender, and delivery route).
All data was recorded in tables using the Microsoft Excel 2007 (Microsoft, Redmond, WA, USA). Statistical analysis was performed by using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). In order to select cases for comparison, a propensity score matching model was used to identify cases matched by pre-pregnancy BMI and gestational age at delivery (using match tolerance for BMI and gestational age 10 and 1, respectively).
To assess for normality of the continuous data, the Shapiro-Wilk test was used. Continuous data with normal distribution was represented in means with standard deviations and compared with the independent t-test. Data without normal distribution was represented in medians with ranges and compared with Mann-Whitney U-test. All nominal data was represented in percentages and compared with Pearson's chisquare test; with Fischer's exact test being used in cases with any expected frequency to be less than 5. In cases with more than two categories for comparison, Bonferroni correction with adjusted standardized residuals were used to identify specific categories with significantly different proportions than the others. Odds ratios and relative risks were also calculated in order to represent risks according to preeclampsia group. For all statistical analysis, a P-value <0.05 was considered to indicate statistical significance.
Results
After applying inclusion/exclusion criteria, the model provided a total of 184 cases that were considered for analysis: 92 cases with antepartum preeclampsia (21.7% mild preeclampsia and 78.3 severe preeclampsia), and 92 with new-onset postpartum preeclampsia (Fig. 1).
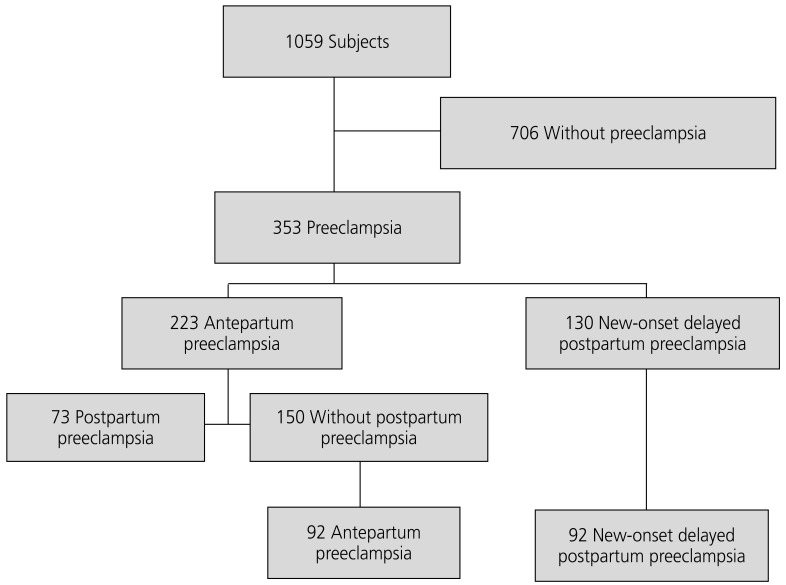
Fluxogram for selection of cases with antepartum and postpartum preeclampsia (n=1,059). Selection of cases with antepartum preeclampsia and new-onset postpartum preeclampsia matched by gestational age and body mass index. The propensity score matching model resulted in 184 matched cases, with 92 in each group.
Demographic data is shown in Table 1. Compared to matched postpartum preeclampsia cases, antepartum preeclampsia presented significantly lower medians of maternal age (21.0 vs. 28.0, U=2,079, P<0.001), gravidity (2.0 vs. 3.0, U=2,843, P<0.001) and parity (0.0 vs. 2.0, U=1,569, P<0.001). Also, insurance was statistically different between groups (χ2(2)=11.468, P=0.003); having less patients with private insurance in the antepartum group (z-score=-3.2, P<0.008). There was no statistical difference in maternal race, past social or medical history between groups.
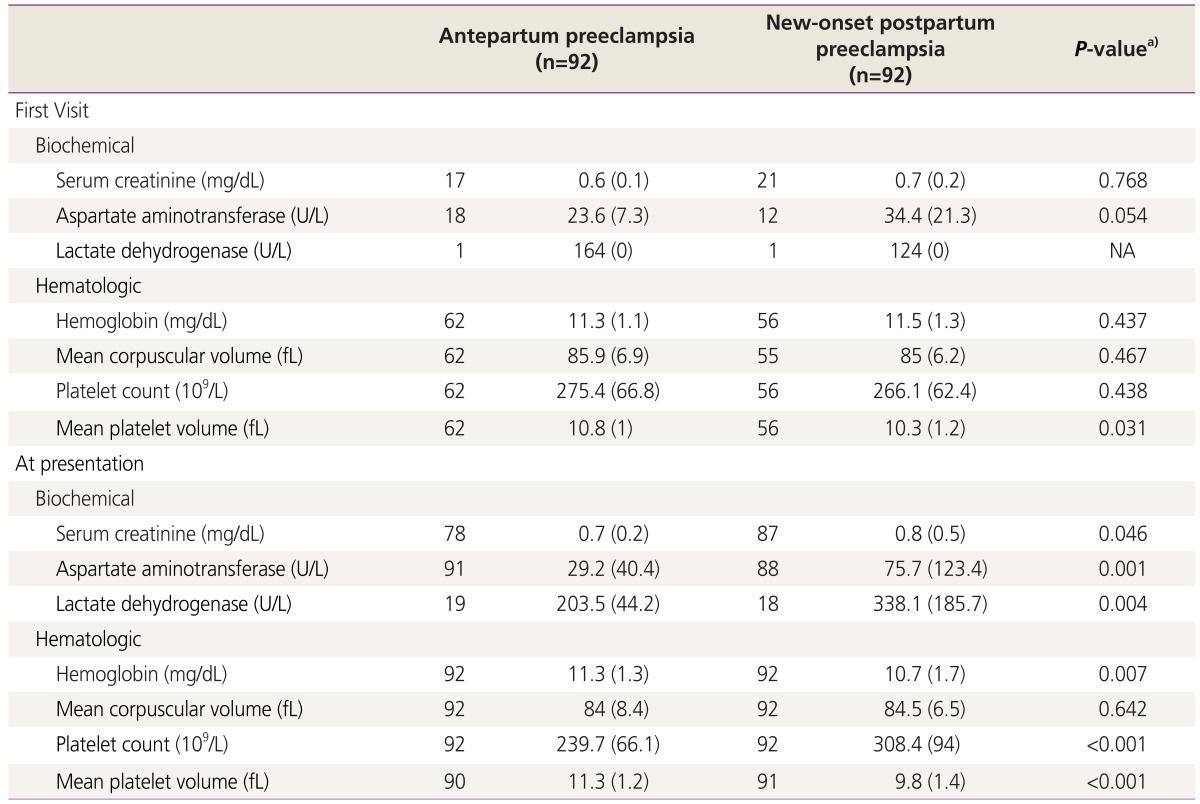
Laboratory markers are shown in Table 2 and Fig. 2. At first visit, there was only statistical difference in the means of platelet volume levels between antepartum and postpartum preeclampsia (10.8 vs. 10.3, t(116)=2.183, P=0.031); there was no statistical difference in the other laboratory markers during the first visit. At presentation, there was statistical difference in the means of serum creatinine (0.7 vs. 0.8, t(163)=-2.009, P=0.046); aspartate aminotransferase (29.2 vs. 75.7, t(177)=-3.412, P=0.001); lactate dehydrogenase (203.5 vs. 338.1, t(35)=-3.071, P=0.004); hemoglobin (11.3 vs. 10.7, t(182)=2.748, P=0.007); platelet count (239.7 vs. 308.4, t(182)=-5.743, P<0.001); and MPV (11.3 vs. 9.8, t(178)=7.839, P<0.001). There was no statistical difference in the mean corpuscular volume. As shown in Fig. 2, the overall variation of the laboratory markers analyzed along pregnancy seem to be more evident in the postpartum preeclampsia group.

Laboratory markers in cases with antepartum and postpartum preeclampsia (n=184). (A) Biochemical markers and (B) hematologic markers. Significant biochemical and hematologic early in pregnancy and at presentation in cases with antepartum preeclampsia and postpartum preeclampsia. Antepartum preeclampsia in black continuous lines, postpartum preeclampsia in black dashed lines. The dots represents the means, the horizontal lines the trend along pregnancy duration, and the vertical lines the confidence intervals of the means. Changes along pregnancy seem to be more evident in postpartum preeclampsia.
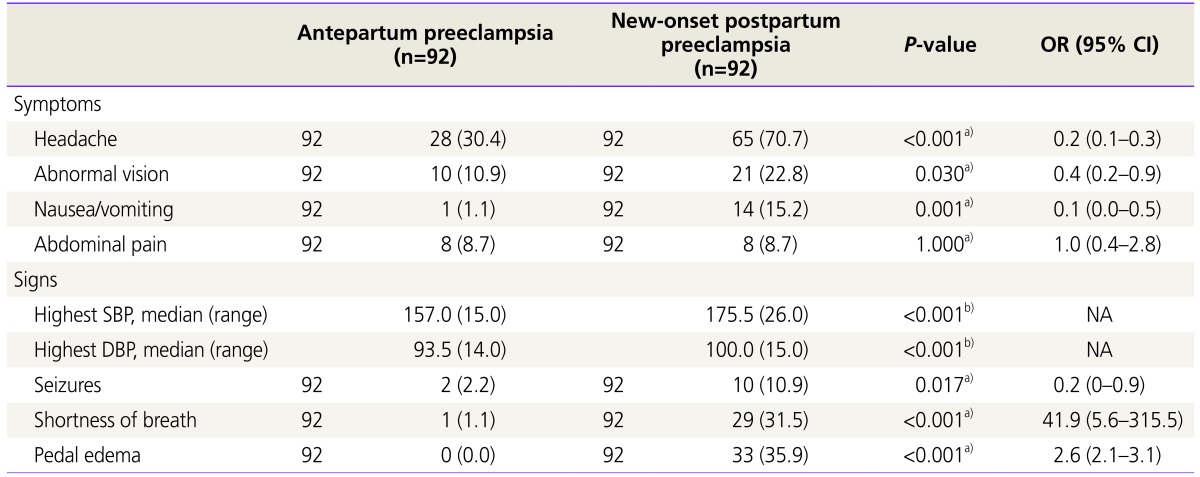
Clinical signs and symptoms at presentation are shown in Table 3 and Fig. 3. Compared to postpartum preeclampsia, antepartum preeclampsia presented with less frequency of headache (30.4 vs. 70.7, χ2(1)=29.764, P<0.001); abnormal vision (10.9 vs. 22.8, χ2(1)=4.694, P=0.030); nausea/vomiting (1.1 vs. 15.2, χ2(1)=12.267, P<0.001); seizures (2.2 vs. 10.9, χ2(1)=5.705, P=0.017); shortness of breath (1.1vs. 31.5, χ2(1)=31.224, P<0.001); and pedal edema (0.0 vs. 35.9, χ2(1)=40.212, P<0.001). Also, antepartum preeclampsia presented with significantly lower systolic (157.0 vs. 175.5, U=1,695, P<0.001) and diastolic blood pressures (93.5 vs. 100.0, U=2,933, P<0.001). There was no statistical difference in the frequency of abdominal pain. As shown in Fig. 3, the overall relative risks of symptoms/signs considered for analysis were higher in the postpartum preeclampsia group.

Clinical presentation of cases with antepartum preeclampsia and postpartum preeclampsia (n=184). Relative risks of significant clinical symptoms and signs in antepartum preeclampsia and postpartum preeclampsia. The black dots represent the relative risks, the black lines the trend along pregnancy duration, the vertical lines the confidence intervals of the means. Overall postpartum preeclampsia presents higher relative risks of symptoms/signs at presentation.
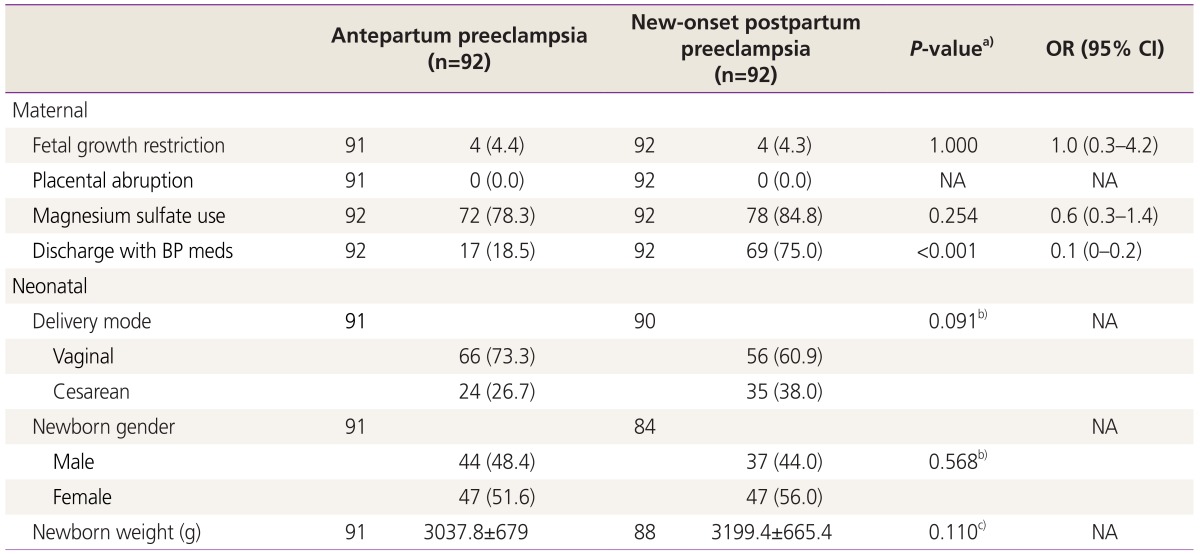
Maternal and neonatal outcome are presented in Table 4. Antepartum preeclampsia required less frequently blood pressure medications upon discharge (18.5 vs. 75.0, χ2(1)=59.034, P<0.001). The other outcomes analyzed were not found to be statistically different.
Discussion
The key findings of this study are that differences between antepartum and new-onset postpartum preeclampsia exist in terms of demographics, clinical presentation and laboratory data which suggests that they both represent different disorders instead of the same disease. Compared to antepartum preeclampsia, patients with postpartum preeclampsia were more commonly older, multiparous and of lower socio-economic status that tended to present clinically with more headache, abnormal vision, nausea/vomiting, seizures, shortness of breath and pedal edema in addition to significantly higher abnormal laboratory markers and blood pressures more commonly requiring anti-hypertensive medication upon discharge.
Yancey et al. [14] reported demographic characteristics of patients with postpartum preeclampsia with a mean age of 28 years, mostly Caucasian and multiparous with history of preeclampsia. Former studies from our institution [5] compared cases with new-onset vs. recurrent postpartum preeclampsia, concluding that these groups are demographically the same, but with lower BMI and lower blood pressures at presentation. Larsen et al. [15] concluded that African-Americans with BMI >30 and antenatal hypertensive disease have a higher likelihood of developing postpartum preeclampsia [15]. The importance of racial disparities in obstetric outcomes has been extensively studied [1617]. Unlike former studies, we define a specific demographic profile of patients that will most likely develop new-onset postpartum preeclampsia and which differs from antepartum preeclampsia: older multiparous woman of poor socio-economic status. Our study confirmed previous findings that women presenting with newonset postpartum preeclampsia were significantly older than women with antepartum preeclampsia in whom blood pressures normalized soon after delivery [18].
Previous authors described that postpartum preeclampsia presents more frequently with headache [511141920], nausea/vomiting [1920] and higher blood pressures [1920] than antepartum preeclampsia. Other studies found little to no clinical differences between study groups [5]. A few studies that examine the development of eclamptic seizures in the post-partum period [41121] concluded that it can present after delivery in a considerable amount of cases. In our series we found that, compared to antepartum preeclampsia, new-onset postpartum preeclampsia presented more frequently with headache, abnormal vision, nausea/vomiting, seizures, shortness of breath, pedal edema and higher blood pressures. The rates of abdominal pain were not statistically different. Differences in the clinical presentation, although it could be explained by postpartum preeclampsia cases presenting with mild symptoms not being admitted, it supports the theory that these conditions might not belong to the same spectrum.
Thrombocytopenia is the most common change in the coagulation system seen in preeclampsia [22]. An initial increase in the MPV can be seen even without a change in the platelet number, displaying earlier signs of platelet compromise in which the count has not yet reached the thrombocytopenic levels [22]. Several studies have shown that the increase in MPV precedes the clinical onset of preeclampsia and might be used as a predictor [2223]. Some other studies suggest that blood pressure correlates with MPV and as such could be an important tool to estimate disease severity [24]. We found that, compared to antepartum preeclampsia, postpartum preeclampsia presented with higher platelet counts and lower MPV. This suggest there might be a different pathogenesis of the latter disorder.
In series related to postpartum preeclampsia, patients were more likely to deliver at term [510] and with newborn birth weights >2,500 g [5]. In our series we did not find statistical differences in newborn birth weights among the antepartum and the postpartum preeclampsia groups.
The weaknesses of our study are intrinsic to its design since as a retrospective cohort chart review, not all of the potential cases could be included as there were situations in which the data was not recorded. Also, important information to include such us weight gain during pregnancy, number of prenatal visits, or duration of symptoms was not collected. Furthermore, since some postpartum cases would only seek medical care if symptomatic, this group would show a higher frequency of complications thus creating some bias in our findings. In addition, errors in data entry or recording is always a possibility in this particular scenario. On the other hand, the main strength lies on the number of collected cases despite the infrequency of new-onset postpartum preeclampsia. A considerable amount of cases compared to prior studies were collected; however, for a case-control study the overall number remains fairly low. Moreover, this is the first study of its kind to compare and identify differences among both disorders that could potentially suggest a different pathophysiologic basis for both conditions.
The importance of researching on the identification of cases with an increased risk of developing postpartum preeclampsia was already illustrated by Sibai [25] who worked on increasing the awareness of post-partum preeclampsia and provided a stepwise approach towards its diagnosis and management. Although it would have been important to provide specific criteria to identify patients that should receive counseling prior to hospital discharge, our study only provided more importantly information to educate primary care providers to identify those patients at risk presenting in the postpartum period with similar complaints. Another study found that women tended to develop eclampsia approximately one week after hospital discharge thus concluding that education about the possibility of developing post-partum preeclampsia should occur prior to hospital discharge irrespective of whether or not patients develop hypertensive disease before discharge [11].
The underlying pathophysiology of new-onset postpartum preeclampsia remains unclear. We could speculate that since it occurs after delivery, this condition is unlikely to belong to the same spectrum of antepartum preeclampsia disorders. It is important to continue studying hypertension during the postpartum period since most patients with postpartum preeclampsia/ eclampsia often present to the emergency department without any history of hypertension during pregnancy or classic features of the disease [14]. Women presenting with postpartum preeclampsia are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism [9]. Therefore, it is important to fully elucidate new-onset postpartum preeclampsia in order to better identify those at increased risk of developing this unexpected condition [1425].
In conclusion, new-onset postpartum preeclampsia has distinctive demographic characteristics, clinical presentation and laboratory data compared to antepartum preeclampsia. These findings suggest that new-onset postpartum preeclampsia may represent a separate clinical entity. Education regarding the profile of patients at risk should be provided to all clinicians caring for women in the postpartum period in order to prevent unwanted complications, particularly emergency room physicians, who are often the first person to evaluate these high risk patients. Further research targeting new-onset postpartum preeclampsia, such as molecular studies to better elucidate the disease mechanism and improved treatment options is warranted.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.