Vasomotor symptoms and the homeostatic model assessment of insulin-resistance in Korean postmenopausal women
Article information
Abstract
The aim of this cross-sectional study was to evaluate the association between vasomotor symptoms (VMS) and insulin resistance, which can be postulated by the homeostatic model assessment (HOMA) index. This study involved 1,547 Korean postmenopausal women (age, 45 to 65 years) attending a routine health check-up at a single institution in Korea from January 2010 to December 2012. A menopause rating scale questionnaire was used to assess the severity of VMS. The mean age of participants was 55.22±4.8 years and 885 (57.2%) reported VMS in some degree. The mean HOMA index was 1.79±0.96, and the HOMA index increased with an increase in severity of VMS (none, mild, moderate and severe) in logistic regression analysis (β=0.068, t=2.665, P =0.008). Insulin resistance needs to be considered to understand the linkage between VMS and cardiometabolic disorders.
Introduction
Vasomotor symptoms (VMS), such as hot flashes and sweating, are thermoregulatory responses resulting from an inability to maintain the body temperature within a specific range [1]. They are some of the most commonly reported symptoms in postmenopausal women, and disturb women at work, interrupt daily activities, and disrupt sleep [2]. We has recently reported that the presence of VMS is associated with the risk of metabolic syndrome in Korean postmenopausal women [3]. Those findings are in line with the several previous studies reporting the association between VMS and worse metabolic conditions or cardiovascular disease risk factors [45678]. In contrast, only a few studies have evaluated the relationship between VMS and insulin resistance. Thurston et al. [9] reported the association between hot flashes and insulin resistance by using the homeostatic model assessment (HOMA) index in a US national cohort study. To date, no study has evaluated these associations in the Korean population because of a lack of attention to the significance of such associations.
The aim of this study was to evaluate the association between VMS and insulin resistance, which can be postulated by fasting glucose levels and HOMA index, in Korean postmenopausal women.
Materials and methods
This cross-sectional study included 2,457 Korean postmenopausal women aged 45 to 65 years who were self-referred for a routine health checkup at the Korea University Anam Hospital (Seoul, Korea) between January 2010 and December 2012. Postmenopausal status was defined as at least 12 consecutive months of amenorrhea with no other medical cause. Each participant provided written informed consent, and this study was approved by the institutional review board of the Korea University Medical Center. The exclusion criteria were as follows: lack of information on menopausal symptoms; lack of data of fasting insulin levels and HOMA index; current hormone use; current medication for diabetes mellitus or dyslipidemia; depressive mood disorders; overt thyroid disorder; surgical menopause; bilateral oophorectomy; past history of chemotherapy or pelvic radiotherapy due to malignant disease; presence of cardiovascular disease such as prior myocardial infarction, angina, stroke, and peripheral arterial diseases; and presence of chronic diseases such as renal failure, liver cirrhosis, and current infectious diseases. Finally, 1,547 postmenopausal women were included in our analysis.
All participants were required to complete an menopause rating scale (MRS) questionnaire, the menopause-specific quality-of-life scale, which was developed to measure the severity of menopause-related complaints by rating a profile of symptoms [10]. To access the severity of VMS, we used a MRS 1 questionnaire. A 5-point rating scale allows the patient to describe the perceived severity of symptoms (none, 0; mild, 1; moderate, 2; severe, 3; and extremely severe, 4) by checking the appropriate box. And for the statistical analyses, extremely severe (4) combined with severe (3), because of small number of women with extremely severe VMS. Thus, we could divide all participants into four subgroups according to the severity of VMS, as following: none (MRS 1 score, 0), mild (1), moderate (2), and severe (3 and 4).
All blood samples were obtained at 9:00 AM after overnight fasting. Fasting blood sugar (FBS), total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride (TG) levels were measured using standard clinical chemistry methods. Insulin level was determined via radioimmunoassay using a commercially available kit from Biosource (Biosource Europe SA, Nivelles, Belgium). HOMA, an index for assessing insulin resistance from fasting glucose and insulin concentrations [11], was calculated using the formula [(fasting insulin×fasting glucose)/405], because the glucose level was measured in mass units (mg/dL). Alcohol intake was categorized according to frequency of alcohol consumed per week as follows: none or one time versus more than one time per week. Physical exercise was categorized according to the frequency of activity lasting at least 20 minutes per day: none or one time versus more than one time per week. Current smoking was defined as current cigarette smoking at the time of the visit.
Continuous variables are presented as mean with standard deviations, and categorical variables are expressed as counts and percentages. Metabolic characteristics of the subgroups divided by the severity of VMS were compared using the ANOVA test with Bonferroni post-hoc test for continuous variables and Pearson's χ2 test for categorical variables. Statistical analysis for linear trends between the severity of VMS and HOMA index was conducted using linear regression analysis method. All statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). All P-values were based on 2-sided tests, with statistical significance defined as P <0.05.
Results
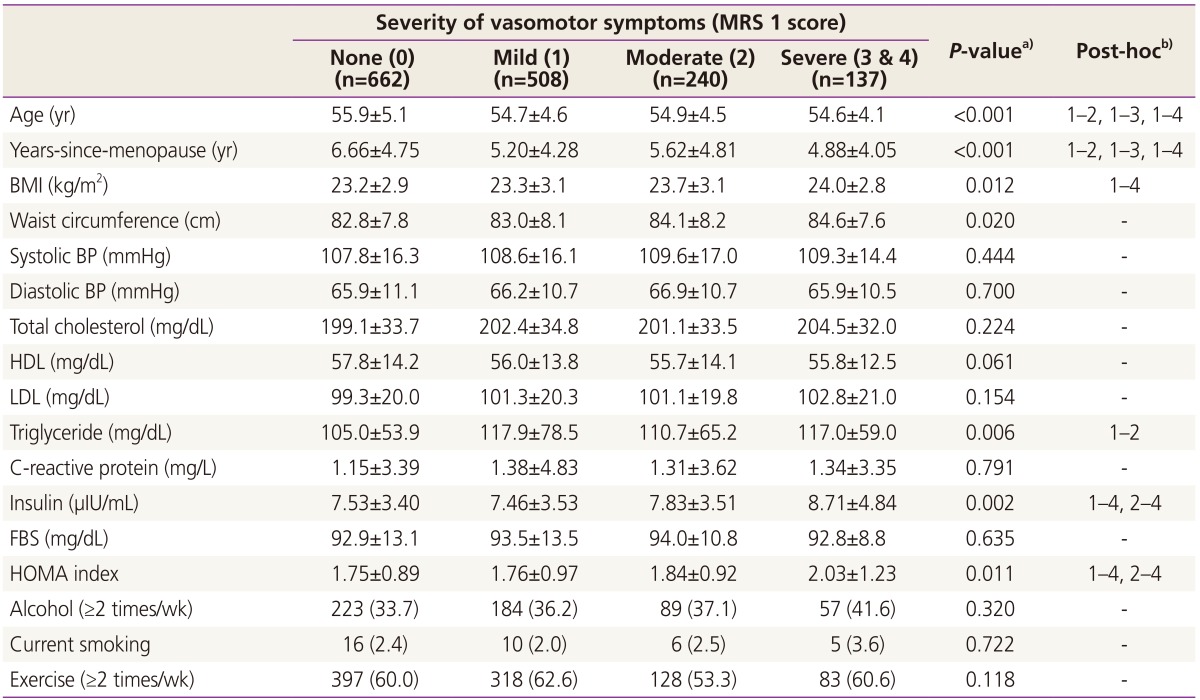
A total of 1,547 postmenopausal women (mean age, 55.2±4.8 years) were included in this study. We reviewed data including the possible factors of metabolic syndrome and VMS: age, age at menopause, body mass index, systolic blood pressure, diastolic blood pressure, waist circumference, C-reactive protein, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, TG, insulin, FBS levels, HOMA index, alcohol intake, current smoking and physical exercise level. The participants were not obese (body mass index, 23.4±3.0) or hypertensive (systolic blood pressure, 108.5±16.2; diastolic blood pressure, 66.2±10.9) and were not diabetic (insulin, 7.7±3.6; FBS, 93.3±12.6). We divided the patients into several groups according to the severity of their VMS (none, mild, moderate, and severe), and metabolic characteristics of those subgroups are presented in Table 1. Women with any degree of VMS were younger and had shorter duration after menopause than women without VMS. Women with severe VMS were more obese than women without VMS, and had higher HOMA index and fasting insulin levels than women without VMS or women with mild VMS (Table 1).
Fig. 1 show that the HOMA index tended to increase in participants reporting higher scores on MRS 1. Logistic regression analyses were performed to assess the linear association between VMS and insulin resistance. The HOMA index seemed to increase with an increase in the MRS 1 scores which represent the severity of VMS (β=0.068, t=2.665, P =0.008).

Trends in homeostatic model assessment (HOMA) index based on the severity of vasomotor symptoms (VMS). Statistical analyses for linear trends were conducted using univariate linear regression analysis method.
After adjusting for confounding factors including age, years-since-menopause, body mass index, TG, however, there was no remained statistical significance of relationship between HOMA index and severity of VMS on multivariate regression analyses (β=0.032, t=1.376, P =0.169).
Discussion
We evaluated the association between menopausal symptoms and insulin resistance in postmenopausal women. In this study, the HOMA index significantly increased in postmenopausal women with high scores on MRS 1. To the best of our knowledge, this study is the first to report an association between insulin resistance and VMS in Korean postmenopausal women. Few previous studies have focused on this association. In a study of 3075 US women in menopausal transition, hot flashes were associated with an increased HOMA index [9]. And some studies are now focusing on the relationship between VMS and cardiovascular diseases or metabolic syndrome. Our previous study based on data obtained from the same population with present study revealed that the presence of VMS is associated with the risk of metabolic syndrome in postmenopausal women [3].
Insulin resistance is a pathological condition characterized by decreased sensitivity of peripheral tissues to insulin which is the pathological basis for diabetes and several cardiovascular diseases [12]. Estrogen is shown to influence insulin receptor expression on those tissues, especially on adipocytes, thus its' level is significantly associated to insulin resistance and metabolic syndrome [1314]. Given that the appearance of VMS may affect estrogen deprivation [15], changes in circulating estrogen levels during peri- or postmenopausal period might work as link between the VMS and insulin resistance. However, data of circulating estrogen levels were not available in present study. On the other hands, insulin resistance is pervasive features of obesity, increasing with weight gain and diminishing with weight loss [16]. An increase in the total and abdominal adiposity also can raise core body temperature and it may increase the occurrence and severity of VMS [17]. Thus obesity might be an important shared risk factor of VMS and insulin resistance. Third, the possibility of the role of the physiological system cannot be ruled out. The sympathetic predominance of VMS might alter the glucose availability and pancreatic insulin production [9]. The hypothalamic-pituitary-adrenal axis may play a role between both conditions [18]. All of these multiple mechanisms, which require further studies, may explain the association between VMS and insulin resistance.
VMS are the main indication for menopausal hormone therapy (MHT) in postmenopausal women. An important implication can be drawn from this statement. If VMS are significantly associated with insulin resistance, as shown in our study, we can postulate that women with insulin resistance would have a greater need for MHT than those without insulin resistance. From this context, the possibility of the curative effect of MHT in patients with insulin resistance should be considered. One meta-analysis reported the effects of MHT in women with type 2 diabetes [19]. In this study, pooled results of 107 trials showed that MHT reduced abdominal fat, HOMA index, and new-onset diabetes in women without diabetes. The importance of the relationship between VMS and insulin resistance and the possibility of MHT use for the treatment of postmenopausal women with diabetes are understood in this context. On the contrary, it is theoretically possible that MHT in women with severe VMS may make the cardiovascular risks worse because women with insulin resistance are likely to have more atherosclerosis [20].
In conclusion, our results suggest that VMS in postmenopausal women are associated with increased insulin resistance. VMS and insulin resistance are associated with each other in many metabolic aspects. Thus, clinicians should pay more attention to postmenopausal women complaining of VMS, because they can be considered to be at high risk of many types of metabolic diseases.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.