|
 |
- Search
| Obstet Gynecol Sci > Volume 56(1); 2013 > Article |
Abstract
Objective
The aim of this study was to evaluate the prognostic value of serum CA-125 in advanced epithelial ovarian cancer with complete remission after primary adjuvant chemotherapy.
Methods
We reviewed the records of 120 patients with advanced epithelial ovarian cancer who underwent primary surgery followed by adjuvant therapy at our institution between January 1998 and December 2005.
Results
The median progression free survival was 21.6 months and 12.5 months in patients with nadir CA-125 levels Ōēż10 U/mL and 10 to 35 U/mL, respectively. Median overall survival in the same respective order was 130.2 months and 35.3 months. The level of serum CA-125 after the first cycle of adjuvant chemotherapy was most significantly higher in the recurrent group compared with the non-recurrent group. The optimal cut point of CA-125 on the receiver operating characteristic curve was 35 U/mL. Median progression free survival was 64.6 months and 12.8 months in patients with nadir CA-125 levels Ōēż35 U/mL and >35 U/mL, respectively, after first cycle of adjuvant chemotherapy.
Ovarian cancer is the leading cause of gynecological cancer death in women in the United States, causing an estimated 13,850 deaths in 2010 [1]. Unfortunately, due to the absence of detectable symptoms in the early stage of the disease and a lack of an effective screening method, approximately 75% of women with ovarian cancer present with stage III and IV [2]. The 5-year relative survival rate of ovary cancer patients is 89.3% and 65.5% for stage I and II, respectively, whereas the rate is only 33.5% and 17.9% for stage III and IV, respectively [3]. In the last two decades, only stage I and II patients showed improvement in cure rate. In contrast, stage III and IV patients showed prolongation in survival time but no improvement in survival rate [4]. Age, performance status, stage of the disease at diagnosis, extent of cytoreductive surgery and residual disease are important independent prognostic factors for survival [5,6].
Many researchers have investigated whether the CA-125 level can be correlated to cancer relapse and survival in various clinical situations. Lower levels at completion of primary therapy [7], lower levels prior to maintenance therapy [8], early changes during maintenance therapy [9] and early normalization during primary chemotherapy [10] could predict positive tumor response and longer survival. In addition, nadir levels of serum CA-125 during primary chemotherapy could predict the risk of tumor recurrence [11,12].
Markman et al. [13] reported that early changes in CA-125 levels are an independent prognostic factor of survival. In their study, patients who achieved normalization of serum CA-125 levels (<35 U/mL) after the second cycle of platinum-based chemotherapy had significant improvement in median survival compared with those with serum CA-125>35 U/mL. Rocconi et al. [10] compared patients who achieved normalization of CA-125 by the third cycle of chemotherapy with patients who failed to achieve normalization by the third cycle. Patients with early normalization demonstrated improved progression free survival (PFS), overall survival (OS) and platinum sensitivity.
Recently, it has been suggested that nadir serum CA-125 levels are independently associated with PFS in patients with complete remission after first-line therapy in ovarian cancer [11,12,14]. The optimal cut-off value of serum CA-125 to predict recurrence varied, ranging from 10 to 25 U/mL.
Because there are no reports about the value of serum CA-125 levels in patients with advanced epithelial ovarian cancer with complete remission after chemotherapy with paclitaxel (175 mg/m2) and carboplatin (area under the curve of 5) every 3 weeks for a total of six cycles, we analyzed the value of serum CA-125 levels in this study.
From 1 January 1998 to 31 December 2005, all patients treated for advanced epithelial ovarian cancer at the Department of Obstetrics and Gynecology of Chonnam National University Hospital (CNUH) were identified from the tumor registry database. All patients were screened retrospectively for their initial serum CA-125 level, age at diagnosis, tumor histology and grade, stage of disease based on the International Federation of Gynecology and Obstetrics (FIGO) staging system, availability of serial serum CA-125 level determinations and timing of recurrence and/or disease status at last follow-up. Patients with epithelial tumors of borderline malignancy, germ cell tumor and gonadal stromal tumor were excluded from this investigation.
Following initial eligibility screening, the following inclusion criteria were applied to determine the final study population: 1) elevated (Ōēź 35 U/mL) serum CA-125 level at the time of diagnosis, 2) histologic documentation of epithelial ovarian cancer, 3) stage of disease III (A, B, and C) and IV treated with primary cytoreductive surgery and postoperative chemotherapy, 4) complete clinical and radiographic response to initial treatment with normalization of CA-125 (<35 U/mL), 5) follow-up serum CA-125 determinations and/or radiographic scans at 1- to 3-month intervals, and 6) clinical and radiographic determination of disease status at the time of last follow-up or recurrence.
After primary surgical staging procedure, all patients received combination chemotherapy with paclitaxel (175 mg/m2 by 3 hour infusion) and carboplatin (area under the curve of 5) every 3 weeks for a total of six cycles. The treatment response was assessed by a complete history, physical examination, appropriate imaging studies (chest X-ray, computed tomography, magnetic resonance imaging and positron emission tomography) and serum CA-125 level. Serum CA-125 levels were measured preoperatively and within 1 week before each cycle of primary adjuvant chemotherapy. All serum samples were analyzed in the same laboratory at CNUH. Optimal cytoreductive surgery was defined as a following review of the surgery and pathology report <1 cm of residual disease was present (following the recommendations of the Gynecology Oncology Group Protocol 17). Complete response was defined as the absence of all measurable disease for at least 4 weeks and the normalization of the serum CA-125 level.
Overall differences in the serum CA-125 level according to the nadir serum CA-125 level were assessed by Student's t-test and the Wilcoxon rank-sum test. A receiver operating characteristic (ROC) curve was used to determine the most clinically useful serum CA-125 threshold value to predict a recurrence. To estimate linear correlation between nadir CA-125 levels and clinical variables, simple lineal regression analysis was used. A multivariate Cox proportional hazards model for OS and PFS was used to assess differences in outcome on the basis of nadir serum CA-125 levels, including other variables, such as age (>65 years vs. Ōēż65 years), stage (IV vs. III), tumor grade (3 vs. Ōēż2), histology (serous vs. non-serous) and residual tumor size. Step-wise regression techniques were used to build multivariate models using a significance level of 0.10 to remain in the model. The differences in OS and PFS by nadir serum CA-125 levels and normalization timing were evaluated using the Kaplan-Meier method with log-rank tests. Statistical analyses were performed using SPSS ver.17.0 (SPSS Inc., Chicago, IL, USA). All P-values reported are two-sided, and a P-value <0.05 was considered statistically significant.
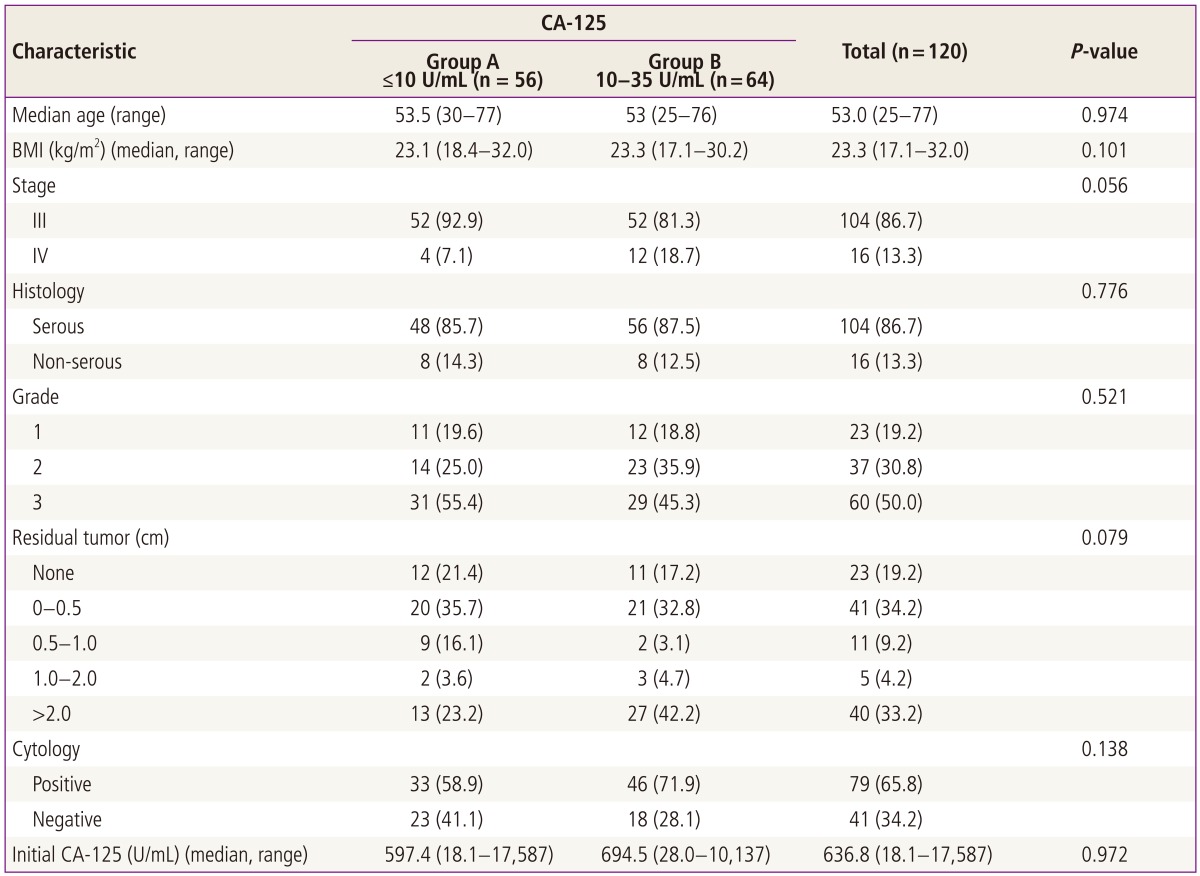
The characteristics of the patients are summarized in Table 1. In the entire study population, the median value of the nadir serum CA-125 level was 10.2 U/mL (range, 0.75 to 34.9 U/mL). The nadir CA-125 level was categorized into two groups: group A, Ōēż10 U/mL (n=56, 46.7% of total) and group B, 10 to 35 U/mL. The median age, body mass index, tumor stage, tumor histology, tumor grade, distribution of residual tumor size, peritoneal cytology and initial serum CA-125 level were not statistically different between the groups.
We evaluated the potential correlation between the residual tumor size and the nadir CA-125 level; non-significant correlation was evident (Table 2). We also tested the correlation between initial serum CA-125 level and the nadir CA-125 level, but it also showed non-significant correlation (P=0.368) (Table 2).
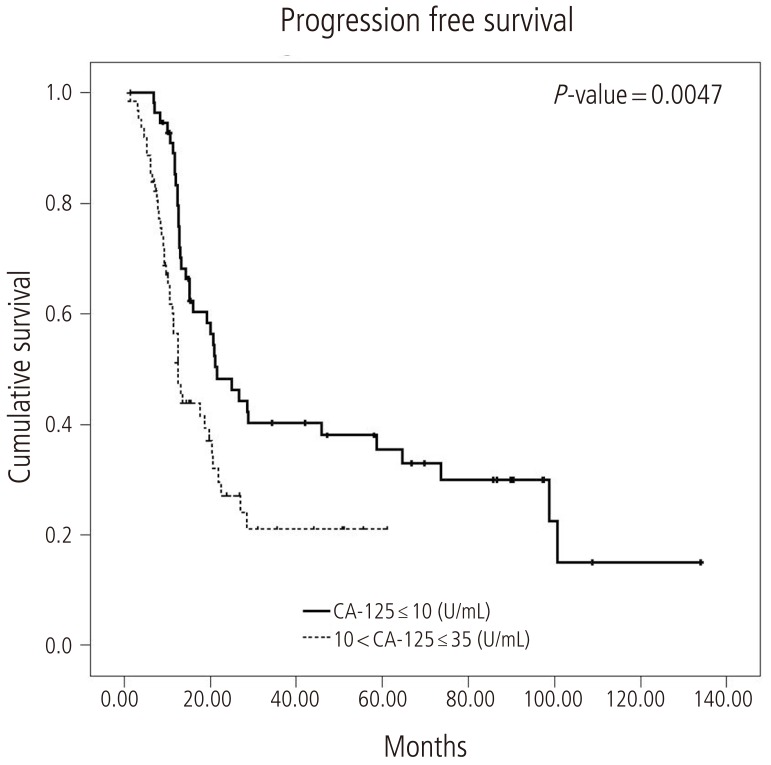
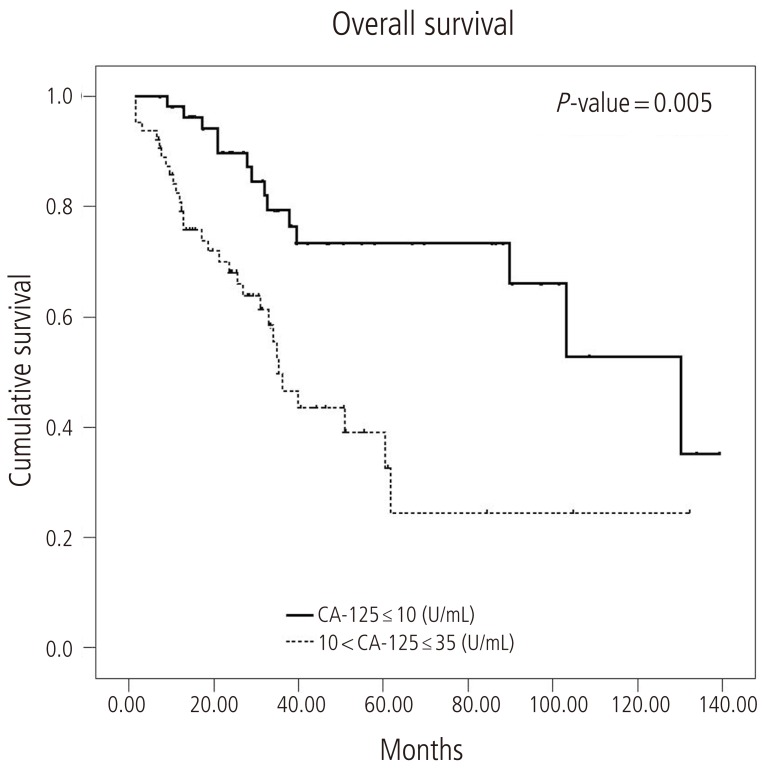
The median PFS in group A was 21.6 months (95% confidence interval [CI], 14.0 to 29.2) and in group B was 12.5 months (95% CI, 10.5 to 14.5) (log-rank test, P=0.0047) (Fig. 1). The median OS was 130.2 months in group A (95% CI, 86.2 to 174.3) and 35.3 months (95% CI, 28.4 to 42.5) in group B (log-rank test, P=0.0005) (Fig. 2).
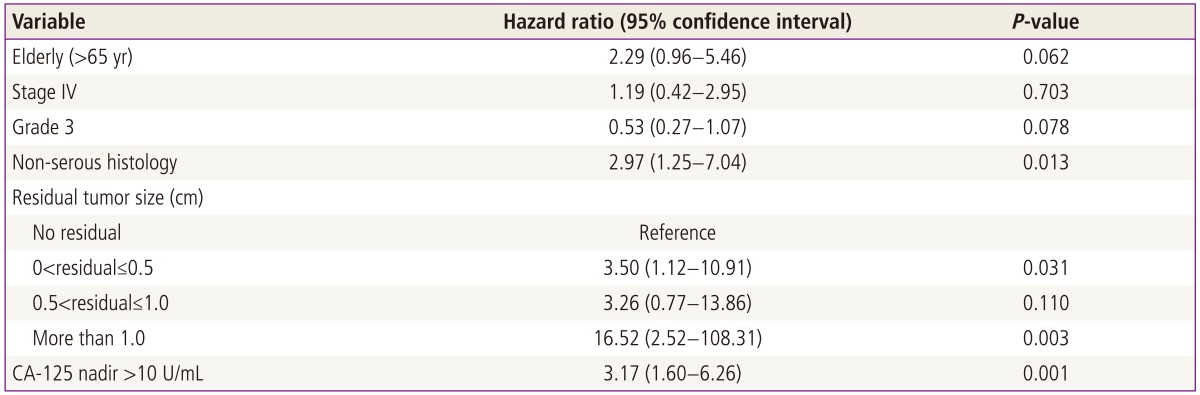
In the multivariate Cox regression model, the hazards ratio was adjusted for covariates. PFS was significantly associated with non-serous histology and residual tumor size >1.0 cm (P=0.013 and P=0.03, respectively) (Table 3). Other variables, such as tumor stage, grade and age at the time of diagnosis were not significantly associated with PFS.
The serum CA-125 levels after surgery and after each of the three cycles of adjuvant chemotherapy were significantly higher in the recurrent group compared with the non-recurrent group (P=0.037, P<0.001, P=0.003, and P=0.032; respectively). ROC curves after surgery and after each of the three cycles of adjuvant chemotherapy revealed that the most significant factor to predict recurrence was serum CA-125 level following the 1st cycle of chemotherapy (area under curve=0.684, P=0.02) (Table 4). The optimal cut point on the ROC curve of serum CA-125 level after 1st cycle of chemotherapy closest to the left upper corner corresponded to a threshold serum CA-125 level of 35 U/mL. At this cut point, the serum CA-125 level after the 1st cycle of adjuvant chemotherapy was able to predict disease progression with a sensitivity of 79.7% and a specificity of 61.0%. The median PFS was 64.6 months (95% CI, 13.9 to 115.2) and 12.8 months (95% CI, 11.8 to 13.8) in patients with serum CA-125 levels Ōēż35 U/mL and >35 U/mL after he 1st cycle of adjuvant chemotherapy, respectively (log-rank test, P=0.0001).
Median value of preoperative CA-125 in this study group was 636.8 U/mL. The median PFS and OS were not statistically different between the patients with preoperative CA-125 levels Ōēź636.8 U/mL and patients with values exceeding 636.8 U/mL (log-rank test, P=0.719 and P=0.316; respectively).
The serum CA-125 level is elevated most consistently in advanced epithelial ovarian cancer, and its value has been demonstrated as a marker for patient prognosis, disease progression and response to chemotherapy. The nadir CA-125 is defined as lowest point reached during treatment. Recently, some researchers reported the prognostic value of nadir CA-125 in advanced epithelial ovarian cancer [11,12,14-16]. The optimal cutoff level of the nadir CA-125 to predict PFS varies from 10 to 25 U/mL. There has been no unified consensus about the fixed cut-off nadir value for CA-125.
In this study, we analyzed serum CA-125 levels in patients who achieved complete remission after primary adjuvant chemotherapy in advanced epithelial ovarian cancer. The median nadir CA-125 level in our subjects was 10.18 U/mL. PFS and OS showed significant differences between patients with nadir CA-125 Ōēż10 U/mL and 10 to 35 U/mL. Similar to a recent study [15], our data revealed that serum CA-125 in patients with advanced epithelial ovarian cancer with complete remission after primary adjuvant chemotherapy is an independent prognostic factor.
Previous studies reported the timing of normalization of CA-125 correlates to survival. Markman et al. [13] reported that early changes in the serum CA-125 level 8 weeks after initiation therapy was a significant prognostic factor on OS in advanced ovarian cancer. Rocconi et al. [10] found that the timing of normalization of CA-125 throughout primary treatment confirmed the aforementioned findings and corresponded to survival, especially in the 3rd cycle of chemotherapy as a clinically useful parameter for response to current therapy. But, there has been little consensus concerning the timing and which level of serum CA-125 is more effective to predict the recurrence. In our study, we analyzed comparison of series serum CA-125 between the recurrent and non-recurrent groups during primary adjuvant chemotherapy. Serum CA-125 levels after surgery and after the 1st, 2nd, and 3rd cycles of adjuvant chemotherapy were significantly higher in the recurrent group compared to the non-recurrent group. The ROC curve yield for estimating the prognostic value of each cycle of CA-125 revealed that the serum CA-125 after one cycle of chemotherapy was the most significant prognostic factor.
The role of preoperative CA-l25 levels as an independent prognostic factor in epithelial ovarian cancer has been investigated [17,18]. Cooper et al. [17] reported that, after adjusting for covariates, there was a significant association between CA-125 levels and disease-specific survival. We analyzed the preoperative CA-125 level to estimate the prognostic value of survival. Patients were divided into two groups by median preoperative CA-125 levels (636.8 U/mL). PFS and OS did not differ significantly between the two groups, with the log-rank P-value being were 0.719 and 0.316, respectively.
Presently, the serum nadir CA-125 level and serum CA-125 level after the 1st cycle of adjuvant chemotherapy were strong independent prognostic factors for advanced epithelial ovarian cancer after complete response.
Early response to adjuvant chemotherapy and normalized serum CA-125 level <10 U/mL were associated with significantly improved prognosis in patients with advanced epithelial ovarian cancer with complete remission. We recommend that, even for patients with advanced epithelial ovarian cancer achieved complete remission, if serum CA-125 levels after the 1st cycle of adjuvant chemotherapy and before 2nd cycle of chemotherapy exceed 35 U/mL and the nadir serum CA-125 exceeds 10 U/mL, closer follow-up is needed, such as frequent imaging study and short follow-up interval.
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. PMID: 20610543.


2. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO Committee on Gynecologic Oncology. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. Int J Gynaecol Obstet 2000;70:209-262. PMID: 11041682.


3. Kosary CL. Cancers of the ovary. In: Ries LA, Young JL, Keel GE, , editors. SEER survival monograph: cancer survival among adults: US SEER program, 1988-2001, patient and tumor characteristics. Bethesda (MD): National Cancer Institute; 2007. p. 133-144.
4. Engel J, Eckel R, Schubert-Fritschle G, Kerr J, Kuhn W, Diebold J, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer 2002;38:2435-2445. PMID: 12460789.


5. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 2009;115:1234-1244. PMID: 19189349.


6. Omura GA, Brady MF, Homesley HD, Yordan E, Major FJ, Buchsbaum HJ, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol 1991;9:1138-1150. PMID: 1904477.


7. Juretzka MM, Barakat RR, Chi DS, Iasonos A, Dupont J, Abu-Rustum NR, et al. CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol Oncol 2007;104:176-180. PMID: 16996584.


8. Markman M, Liu PY, Rothenberg ML, Monk BJ, Brady M, Alberts DS. Pretreatment CA-125 and risk of relapse in advanced ovarian cancer. J Clin Oncol 2006;24:1454-1458. PMID: 16549840.


9. Liu PY, Alberts DS, Monk BJ, Brady M, Moon J, Markman M. An early signal of CA-125 progression for ovarian cancer patients receiving maintenance treatment after complete clinical response to primary therapy. J Clin Oncol 2007;25:3615-3620. PMID: 17704410.


10. Rocconi RP, Matthews KS, Kemper MK, Hoskins KE, Huh WK, Straughn JM Jr. The timing of normalization of CA-125 levels during primary chemotherapy is predictive of survival in patients with epithelial ovarian cancer. Gynecol Oncol 2009;114:242-245. PMID: 19447480.


11. Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol 2005;16:47-50. PMID: 15598937.


12. Prat A, Parera M, Peralta S, Perez-Benavente MA, Garcia A, Gil-Moreno A, et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol 2008;19:327-331. PMID: 18065408.


13. Markman M, Federico M, Liu PY, Hannigan E, Alberts D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol Oncol 2006;103:195-198. PMID: 16595148.


14. Pignata S, Perrone F, Di Maio M, Gallo C, De Placido S. In reply. J Clin Oncol 2005;23:2436-2437.


15. Kang WD, Choi HS, Kim SM. Value of serum CA125 levels in patients with high-risk, early stage epithelial ovarian cancer. Gynecol Oncol 2010;116:57-60. PMID: 19818996.


16. Kang S, Seo SS, Park SY. Nadir CA-125 level is an independent prognostic factor in advanced epithelial ovarian cancer. J Surg Oncol 2009;100:244-247. PMID: 19267361.


17. Cooper BC, Sood AK, Davis CS, Ritchie JM, Sorosky JI, Anderson B, et al. Preoperative CA 125 levels: an independent prognostic factor for epithelial ovarian cancer. Obstet Gynecol 2002;100:59-64. PMID: 12100804.


18. Ivanov S. Epithelial ovarian cancer: preoperative assessment of Ca 125 levels as an independent prognostic factor. Akush Ginekol (Sofiia) 2003;42:16-19. PMID: 12858485.

-
METRICS

-
- 4 Crossref
- 7,372 View
- 34 Download
- Related articles in Obstet Gynecol Sci
-
The analysis of prognostic factors in patients with epithelial ovarian cancer.2006 March;49(3)