Gynecologic malignancy in pregnancy
Article information
Abstract
Gynecologic malignancy during pregnancy is a stressful problem. For the diagnosis and treatment of malignancy during pregnancy, a multidisciplinary approach is needed. Patients should be advised about the benefits and risk of treatment. When selecting a treatment for malignancy during pregnancy, the physiologic changes that occur with the pregnancy should be considered. Various diagnostic procedures that do not harm the fetus can be used. Laparoscopic surgery or laparotomy may be safely performed. The staging approach and treatment should be standard. Systemic chemotherapy during the first trimester should be delayed if possible. Radiation therapy should preferably start postpartum. Although delivery should be delayed preferably until after 35 weeks of gestation, termination of pregnancy may be considered when immediate treatment is required. Subsequent pregnancies do not increase the risk of malignancy recurrence.
Introduction
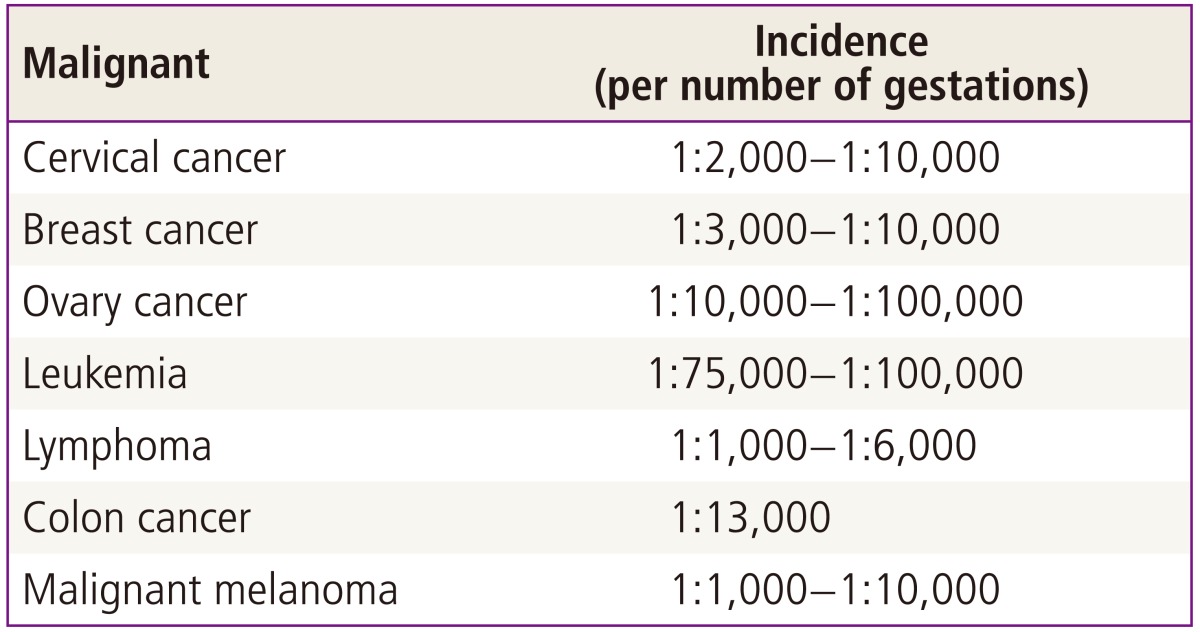
Cancer is a major public health issue. The diagnosis of cancer in pregnancy is a challenge for the clinician, the woman, and her fetus. In several studies, the term "gestational cancer" includes not only cancer diagnosed during pregnancy but also during the first year postpartum. The incidence of cancer during pregnancy is not easy to analyze because of the lack of central registries. However, cancer in pregnancy is fortunately uncommon. Some studies have reported an incidence of gestational cancer as low as, 0.02% to 0.1% [1-3], and it is lower in developing countries because of the younger age of pregnant women [4]. Cancer diagnosed during pregnancy has become more frequent over the last 3 decades, because the number of women childbearing at an older age is increasing (Table 1). This current trend to delay pregnancy has increased the occurrence of pregnancy-associated cancer [5].
Physician expertise and multidisciplinary care are both required for the appropriate treatment of gestational cancer. The gynecological oncologist should assist the consultation between the obstetrician and the medical and radiation oncologists to determine any issues that may arise during the treatment of the patient. The psychological effect of this condition on the patient can often result in improper responses from the patient and the clinician as well as additional medical problems [5].
Most cancers diagnosed during pregnancy are cervical and breast cancer, accounting for 50% of all gestational cancers. Approximately 25% of malignant cases diagnosed during pregnancy are hematological (leukemia and lymphoma). Cancers occurring less frequently during pregnancy include ovarian cancer, thyroid cancer, colon cancer and melanoma [4]. A recent investigation reported a breast cancer incidence rate is 1 in 7,700 pregnancies [6]. The prognosis is similar to that of non-pregnant patients and, a detailed history and a physical examination should be the basis of the diagnostic work-up. Endoscopies, lumbar punctures and bone marrow aspirations may be performed and are considered low risk for pregnant women. However, during these procedures, sedatives and analgesics should be used with caution. The risk of fetal harm during a biopsy is low. Termination of the pregnancy for the treatment of cancer does not improve the patient's prognosis [5]. Suboptimal diagnosis and treatment will result in an impaired prognosis. We will discuss the different treatment modalities used during pregnancy. In addition, we focused on specific features of gynecological malignancy in pregnancy.
Treatment modalities
1. Surgery in pregnancy
Surgery is needed in 0.75% to 2% of pregnancies. The most common indications for surgery are cholecystitis, appendicitis and ovarian cysts. Anesthesia during pregnancy is considered safe [7]. Fetal effects are more correlated to maternal hypoxia, hypotension, hypothermia or glucose metabolism rather than anesthesia. The risk of miscarriage and congenital anomalies does not increase with surgery. Preterm deliveries usually occurred in cases appeared after abdominal surgery and peritonitis. Since pain may induce premature labor, adequate postoperative use of analgesia is important. Furthermore, prophylaxis for thrombosis is needed [8]. Surgery in the first trimester slightly increases the risk of fetal loss because of general anesthesia [9]. The probable risk for surgical complications is present, although most anesthetic drugs are safe for the fetus [10]. Laparoscopic surgery can be performed during pregnancy by an experienced physician. Open laparoscopy could be helpful to prevent uterine perforation [11,12].
2. Systemic chemotherapy during pregnancy
Chemotherapy exposure during pregnancy increases the risk of fetal damage. The phase of organogenesis is the most vulnerable period for the fetus and occurs from day 10 to week 8 after conception. The risk of major malformations, spontaneous abortions, and fetal death may be increased because of chemotherapy during the first trimester [13,14]. Chemotherapy exposure in the second and third trimester does not cause teratogenic effects; however, the risk for low birth weight and fetal growth restriction may be increased [14]. A study of 376 pregnant women reported the following after uterine exposure to chemotherapy: 5% cases of premature delivery, 7% cases of intrauterine growth restriction, 6% cases of fetal or neonatal death, and 4% cases of transient myelosuppression. Since the hematopoietic system, genitals, eyes, and central nervous system are vulnerable during organogenesis, chemotherapy should be delayed until gestational week 14 [15]. When considering chemotherapy during pregnancy, the effect of delayed treatment on maternal survival should be evaluated. Since the mother as well as the fetus is at risk for infections and bleeding during delivery because of hematological toxicity, chemotherapy should be discontinued 3 to 4 weeks before delivery, especially after 35 weeks of pregnancy [15].
3. Chemotherapeutic agent
The pharmacokinetics and pharmacodynamics of chemotherapy may change because of the physiologic alterations during pregnancy. The physiological changes during pregnancy such as faster hepatic oxidation, increased renal clearance and plasma volume, and enlarged third space are important [15]. The greatest potential for fetal damage is the use of folic acid antagonists such as methotrexate, which is a cytotoxic drug [16]. However, 5-fluorouracil can be often used after organogenesis. Doxorubicin and epirubicin (the antitumor antibiotics) can be used as well [15,16], but fetal loss after idarubicin exposure has been reported [17]. Cyclophosphamide, cisplatin, and carboplatin (alkylating agents) are rather harmless. Even when sensorineural hearing loss after cisplatin use has been reported, confounding factors such as postnatal gentamycin treatment and prematurity are observed [15]. No fetal problems were reported in 11 pregnant women treated with taxanes (5 on docetaxel and 6 on paclitaxel) [18-28]. There are at least 8 known cases pregnant women that received combination chemotherapy with bleomycin, etoposide, and cisplatin for germ-cell tumors [29-35]. However, cerebral atrophy with significant ventriculomegaly was reported in 1 child [32]. Tamoxifen is teratogenic in animals and 10 cases of fetal abnormalities have been reported among 50 pregnant women exposed to it. The use of tamoxifen should be postponed until postpartum [36].
4. Supportive care during chemotherapy
Supportive care after chemotherapy can be provided along with the general recommendations [37]. Most patients may experience emesis and nausea during or after chemotherapy. Antiemetic treatment with antiserotonin drugs, antihistaminic drugs, or metoclopramide is not associated with fetal malformations [38,39]. Pregnant women with neutropenic fever after chemotherapy should be treated with antibiotics. Considerable data are available on fetal safety after the use of erythromycin, cephalosporins, and penicillins [40]. However, sulfonamides should be avoided because of the risk of cardiac malformations and neural tube defects [41]. Hydrocortisone and methylprednisolone are metabolized in the placenta and small amounts can cross into the fetal partition [42]. Antenatal repeated exposure to beta/dexamethasone resulted in hormonal disturbances, delay in maturation, and reduced brain and body burden in animal models [43]. Nonsteroidal anti-inflammatory drugs (NSAID) and acetaminophen are safe [44,45]. However, oligohydramnios, premature closure of the ductus arteriosus, and prolonged gestation are correlated with the effect of prostaglandins [46]. Opioid analgesics are not associated with fetal malformation in humans. However, chronic use may induce neonatal withdrawal signs and symptoms and maternal addiction [47]. Treatment with erythropoietin and granulocyte colony-stimulating factor in chemotherapy-induced cytopenias is safe for both the fetus and the mother [48]. However, since data on the use of recombinant human erythropoietin (rhEPO) during pregnancy are insufficient, it can only be used if blood transfusion is contraindicated [49-51].
5. Targeted treatment
Trastuzumab is a standard drug for the treatment of breast cancer. Trastuzumab triggered oligohydramnios with fetal renal insufficiency in 3 case reports [52]. The use of trastuzumab during pregnancy is limited because it causes fetal renal function changes [53,54].
Currently, cetuximab (Erbitux) and bevacizumab (Avastin) are being used in the treatment of metastatic cancer. However, bevacizumab has an antiangiogenic effect with severe adverse effects in pregnant women. No report is available on these drugs during pregnancy and hence, they should not be administered [15].
6. Radiotherapy during pregnancy
Because radiation can damage the fetus, radiotherapy should be reconsidered. The risk of adverse effects is associated with gestational age, field, and fractionation as well as the radiation dose [55]. Radiation contact with a limited dose of 0.1 to 0.2 Gy during organogenesis, 2 to 8 weeks after conception, could lead to congenital abnormalities [56]. The fetal central nervous system during 8 to 25 weeks after conception is sensitive to radiation, and a radiation exposure dose of >0.1 Gy could decrease the intelligence quotient [57]. Radiation in the second and third trimesters is correlated with carcinogenic effects within the first decade of life, such as the development of solid tumors and leukemia. The Oxford clinical sequences revealed a 6.4% risk of carcinogenesis per Gy of radiation exposure during gestation from childhood to young adulthood [58,59]. Breast cancer during pregnancy can be treated with radiotherapy. Irradiation to the pelvis can result in fetal loss [57]. Pregnant women with cancer should consider alternative therapies such as postponed radiotherapy or neoadjuvant chemotherapy. Therapeutic abortion does not provide a better outcome when appropriate treatment for cancer is given [15,36]. However, termination should be considered when there is a need for immediate treatment of an abdominal-pelvic malignancy.
Imaging and diagnosis during pregnancy
Diagnostic imaging can be performed safely if the radiation dose received by the fetus is >1 mGy. Pelvic computer tomography (CT) exposes the fetus to 10 to 40 mGy of radiation [55]. Positron emission tomography (PET)-CT has been recently shown to be useful for follow-up examinations after cancer treatment. However, since PET-CT may involve the use of higher radiation levels than a CT scan, it cannot be used during pregnancy [60,61]. The use of alternative diagnostic methods including magnetic resonance imaging (MRI) or sonography can sometimes be beneficial. However, gadolinium, which is sometimes used in MRI, crosses the placenta and is associated with fetal malformation in rats. The high-energy radiowave stimulation in magnetic fields may produce fetal cavitation and heating [62]. Some radiologists are opposed to the use of MRI in the first trimester while others recommend against the use of gadolinium; no consensus has been reached [62].
Monitoring pregnancy and neonatal outcomes after cancer therapy
Generally, the fetus and mother should undergo standard prenatal care. Pregnancy-related problems should be managed with the usual obstetrical treatments. The available information on monitoring is limited. Echocardiographic follow-up data in children have shown normal cardiac function after exposure to cyclophosphamide and doxorubicin at 24 weeks. Repeat follow-up echocardiograms are performed until the child is aged 2 years [63]. As previously mentioned, ventriculomegaly and bilateral hearing loss was reported after the use of bleomycin, etoposide, and cisplatin during pregnancy [32,64]. A recent study of 376 cases of in utero exposure to chemotherapeutic agents demonstrated a 5% rate of prematurity, 4% rate of neonatal transient myelosuppression, 1% rate of neonatal death, and 5% rate of intra-uterine death [15].
Cerebral palsy and neurological impairments are associated with preterm birth. Therefore, it is vital to prolong the pregnancy until after 35 weeks as much as possible [65]. After delivery in high risk cases, concomitant placental involvement should be carefully verified. Vertical transmission of malignant tumor is rare; 62 cases have been described. However, fetal involvement has not been reported for gynecological malignancy [66]. Breastfeeding throughout chemotherapy is contraindicated because most chemotherapeutic agents used are passed on to breast milk [16].
Gynecologic malignancy in pregnancy
1. Cervical cancer in pregnancy
Early detection of invasive cervical cancer in pregnant women is possible because the Papanicolaou test, cervical inspections, and pelvic examinations are routinely performed during antenatal care. Therefore, earlier stages of cervical cancer are diagnosed at an operable stage during pregnancy, representing a 2 to 3 fold higher chance compared to the general population [67-69]. The most common symptom is vaginal bleeding apart from pregnancy. However, the bleeding symptom may be mistaken for antepartum hemorrhage due to threatened abortion. Cytological test results during pregnancy may show trophoblast cells or squamous metaplasia that could be misdiagnosed as dysplasia [69-72]. Cervical ectropion during pregnancy allows earlier detection of the transformation zone. However, pregnant women have increased cervical vascularization and volume, glandular hyperplasia, and stromal edema, making colposcopic findings more difficult to understand [72]. Cervical intraepithelial lesions (CIN) II-III can progress to invasive cancer in >5% of cases during pregnancy [73-76]. Gynecologists should consider that excisional procedures might cause profuse hemorrhage. CIN during pregnancy can be managed conservatively and the treatment can be delayed until the postpartum period. Nevertheless, a control colposcopy every 2 to 3 months is recommended during pregnancy because of the possibility of invasive cancer [75,76]. Since episiotomy scar recurrences have been reported, the delivery mode can be changed [77]. There are no reports suggesting that pregnancy worsens cancer prognosis. Survival rates of pregnant and nonpregnant women with invasive cervical cancer are similar [4].
The most common malignancy diagnosed during pregnancy is invasive cervical cancer. The treatment during pregnancy can be selected based on the following considerations: intention to preserve pregnancy, gestational age, and cancer stage. Conization is the treatment of choice in stage IA. Since the visibility of the exocervix is improved during pregnancy, flat cone biopsy could be sufficient [77]. Termination and immediate treatment of cervical cancer is recommended for patients at <20 weeks of gestation. When cervical cancer is diagnosed after 20 gestational weeks, the treatment is postponed until fetal maturation. Final treatment can follow the Cesarean section [77]. There are no randomized-controlled trials about the effects of treatment delay on cancer outcomes. However, the possible risks during cancer treatment should be discussed with the patient. Conservative management should only be considered if there is a strong desire to maintain the pregnancy and if there is enough evidence to suggest that the pregnant woman will not be harmed. If a woman diagnosed with cervical cancer during the first trimester wishes to continue the pregnancy, conservative management is recommended until the second trimester. The methods for pregnancy-sparing management are the same as those for fertility-sparing surgery [78]. A conservative approach can be considered with a negative nodal status. When a nodal biopsy is performed, the pelvic lymph nodes may be misdiagnosed as malignant because of the decidual changes during pregnancy [79-83]. Conization and radical trachelectomy have been performed for the maintenance of the pregnancy. The bleeding risk during procedure increases with the pregnancy period. To reduce the risk of blood loss and abortion, the second trimester, from 14 to 20 weeks, is a favorable time for cervical conization [77]. Radical trachelectomy during pregnancy is very risky and may cause profuse hemorrhage and pregnancy loss [84]. Alternative management strategies such as neoadjuvant chemotherapy can be used to increase protection against cervical cancer after organogenesis is completed (13 weeks of gestation) [85-87]. Guidelines for fertility preservation in women with cervical cancer during pregnancy were suggested in a 2008 international consensus meeting on gynecologic cancers in pregnancy [88]. In a pregnant woman with cervical cancer, if the tumor is <2 cm (stage IA2-IB1), a lymphadenectomy should be perform at first. In the case of negative nodal metastasis, trachelectomy or neoadjuvant chemotherapy followed by trachelectomy may be performed. Standard treatment (radical hysterectomy or concurrent chemoradiation therapy) is administered if nodes are positive. In stage IB1 2-4 cm tumors, lymphadenectomy or neoadjuvant chemotherapy followed by lymphadenectomy may be performed. Preservation of the pregnancy requires negative nodal metastasis and good response to neoadjuvant chemotherapy. For stage IB2-IIB, neoadjuvant chemotherapy is offered until fetal maturity. Standard treatment is considered if there is no excellent response to neoadjuvant chemotherapy. In more advanced stages, classic treatment (radical hysterectomy or radiation therapy) should be given after delivery at the appropriate gestational age (Fig. 1).
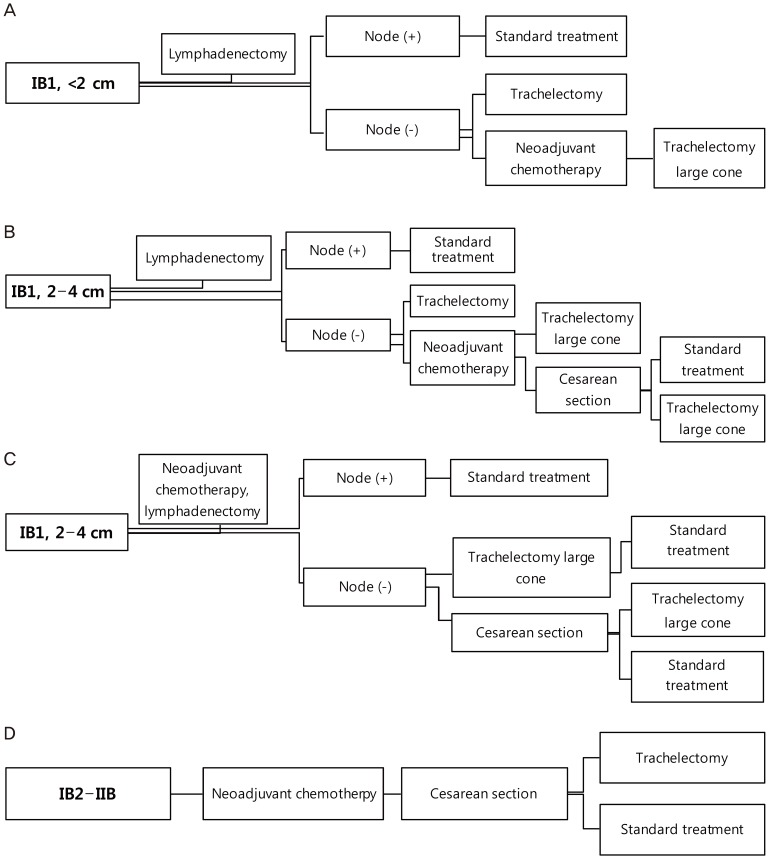
Guidelines of an International Consensus: algorithm of cervical cancer. (A) Cervical cancer stage IB, <2 cm treated during second trimester wishing to preserve the fertility and pregnancy. (B) Cervical cancer stage IB1, 2 to 4 cm treated during second trimester wishing to preserve the fertility and pregnancy: lymphadenectomy. (C) Cervical cancer stage IB1, 2 to 4 cm treated during second trimester wishing to preserve the fertility and pregnancy: neoadjuvant chemotherapy followed by lymphadenectomy. (D) Cervical cancer stage IB2-IIB treated during second trimester wishing to preserve the fertility and pregnancy.
A Caesarean section is preferred for the prevention of scar recurrences at the episiotomy site [89]. When the cervix is free from cervical cancer after treatment, a vaginal delivery is feasible. The recurrence of abdominal section scars has also been reported [90-92].
2. Ovarian cancer in pregnancy
Over 90% of the adnexal masses found in the first trimester disappear spontaneously. Teratomas, cystadenomas, endometriomas, ovarian cysts, and leiomyomas are the most frequent benign lesions. During pregnancy, malignant and borderline ovarian cancers account for 3% to 6% of cases. Ovarian cancer in pregnancy is rare and has occurred in 1 in 15,000 to 1 in 32,000 pregnancies [93].
Diagnosis of an ovarian tumor during pregnancy is not easy. Uterine subserosal myoma, uterine anomalies, and colonic mass are the differential diagnoses. Ovarian pathologic types include germ-cell (6%-40%), epithelial (49%-75%) and sex cord stromal tumors (9%-16%) [94,95]. The diagnostic method mainly depends on ultrasonographic findings because tumor markers during pregnancy are not helpful. The serum levels of CA-125, alpha-fetoprotein, human chorion gonadotrophin, and inhibin fluctuate during pregnancy [96]; therefore, these markers are less useful during pregnancy. Thus, surgery is mainly decided upon by the sonographic findings and clinical course [95].
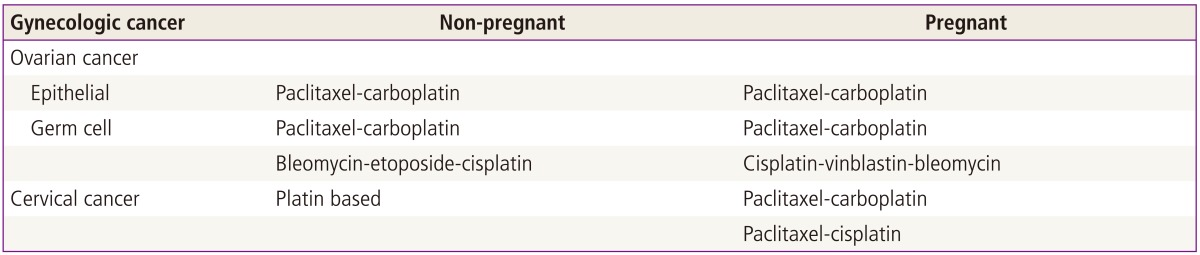
Delaying surgery increases the risk for bleeding, cystic rupture, and torsion. These may be reasons for emergency surgery [95]. Diagnosis and treatment of an ovarian malignant disease may be also postponed. Operating too early can increase the risk for luteal function loss and fetal loss, and late surgery may trigger worse prognosis. Surgical indication is therefore persistent ovarian tumor with a suspicious finding until the second trimester. In the absence of malignancy risk, laparoscopic surgery by an experienced physician is acceptable [95]. Similar to nonpregnant women, a staging operation is needed in pregnant women after the first trimester. Although ovarian cancer in its entirety is rare during gestation, 50% of the cases are germ-cell tumors [97]. Most germ-cell tumors are detected in the early stages. Most epithelial ovarian cancers are also confined to the pelvis [98]. The staging procedure will frequently include washing cytology, ipsilateral salpingooophorectomy, peritoneal biopsies, and omentectomy. Examination of the cul-de-sac and pelvis is commonly suboptimal, since uterine manipulations may be limited to avoid premature uterine contractions. Lymphadenectomy should be performed in selected cases with enlarged nodes during the staging operation. Since germ-cell tumors are chemosensitive tumors, a fertility-sparing surgery is recommended even in the advanced stages if the ovary on the other side is tumor free. However, in epithelial ovarian cancer, a fertility-sparing surgery should only be considered in stage IA/IB, grade 1 [97]. Adjuvant chemotherapy is not needed in International Federation of Obstetrics and Gynecology (FIGO) stage I, dysgerminoma and FIGO stage I immature teratoma [15]. Advanced stage ovarian cancer can be treated in several different ways including primary cytoreductive surgery with termination [99], surgery during pregnancy followed by adjuvant chemotherapy at postpartum, debulking surgery followed by chemotherapy during pregnancy and surgery after delivery, and expectant management [19-25,96,100]. Maintenance of the pregnancy is very difficult when advanced cancer is diagnosed before 20 weeks of gestation. After 20 weeks, maintenance of the pregnancy is possible, but uncertain. As in nonpregnant women, the regimen of choice for neoadjuvant chemotherapy is paclitaxel-carboplatin chemotherapy until fetal maturation. After vaginal delivery or Cesarean section, planned debulking laparotomy may be considered [100]. As mentioned previously, 1 case of ventriculomegaly and cerebral atrophy and 1 case of hearing loss was reported after 1 cycle of bleomycin, etoposide, and cisplatin (BEP) [29-35]. Because of the potential for fetal risk and the high risk of leukemia after the use of etoposide during pregnancy, paclitaxel-carboplatin or cisplatin-vinblastin-bleomycin, instead of BEP, is recommended in pregnant women with germ-cell tumors (Table 2) [101].
3. Vulvar cancers in pregnancy
The most frequent vulvar lesion during pregnancy is human papillomavirus-related vulvar intraepithelial neoplasia (VIN). Surgical excision or laser vaporization is the treatment of choice in VIN. The existing alternatives such as the use of imiquimod or podophyllin are contraindicated, although the use of imiquimod in 1 pregnant woman has been reported [102]. Invasive vulvar cancer during pregnancy is rare. Definitive surgery is considered before 36 weeks of gestation. Invasive vulvar cancer with clinically negative nodes should be handled in the same manner as in nonpregnant women. Standard procedure consists of partial or total vulvectomy and uni- or bilateral inguinofemoral lymph node dissection or sentinel node biopsy [103]. The increased vascularization of the perineum during pregnancy increases the risk of bleeding during surgery. Inguinal node metastasis is a sign of poor prognosis. In the presence of positive inguinal lymph nodes, sufficient treatment is required without break. Cesarean section is decided upon by obstetrical indications [104]. Vulvar melanoma, which is fatal, has been described in pregnant women. Healthy babies have been born even when placental metastasis has been involved [105].
4. Endometrial cancer
Finding endometrial cancer in pregnant women is an extremely uncommon event. Twenty-four cases have been reported. Most cases were diagnosed at abortion or postpartum [106]. Therefore, it is important to confirm the results of the pathology report after curettage.
5. Gestational trophoblastic neoplasms in pregnancy
Gestational trophoblastic disease (GTD) with a coexisting fetus and hydatidiform mole is an uncommon condition and occurs in 1:22,000 to 1:100,000 pregnancies according to several reports [107]. This disease is often complicated by vaginal bleeding, pre-eclampsia, hyperthyroidism, fetal death, and the risk of malignant invasion requiring chemotherapy. Therefore, termination of the pregnancy is often recommended. In pregnant women with GTD, the probability of live birth has been reported to be 40% [108]. However, approximately 20% to 55% of women with benign GTD who maintain their pregnancy develop malignant GTD [107-109].
Conclusion
Although cancer during pregnancy is uncommon, it can be treated effectively without causing fetal damage. A multidisciplinary approach is essential and wide-ranging information for the parents is needed. The maternal outcome will not be improved by terminating the pregnancy. The management strategies must also include the maintenance of quality of life during the current pregnancy and fertility preservation. Every decision related to cancer management should be reached in collaboration with the patients. In many cases, cancer treatment can be postponed until fetal maturation without affecting maternal prognosis. Psychosocial care should focus on active participation of the patient. A lack of consensus might be the reason why perinatologists, oncologists, and gynecologists have little information on each other's fields. Almost 50% of specialists recommend pregnancy termination when cancer is diagnosed. To allow early treatment of cancer, 58% of specialists prefer preterm delivery. Moreover, 37% of specialists will not administer chemotherapy or radiotherapy during pregnancy [110].
Prospective registration studies are rolling and contribution from current practitioners is encouraged. The 'Cancer in Pregnancy' Task Force of the European Society of Gynecological Oncology (ESGO) was founded in 2009 (http://www.esgo.org/Networks/Pages/TaskForces.aspx). Other ongoing studies are http://www.cancerinpregnancy.org (Europe), http://www.germanbreastgroup.de (breast cancer), http://www.cancerandpregnancy.com (US), and http://www.motherisk.org/women/cancer.jsp (Canada).
In conclusion, standard cancer treatment should be recommended to increase maternal chances of survival. Nevertheless, treatment delay should be avoided. Finally, research on the issue of gynecologic malignancy in pregnancy should be the focus.
Notes
No potential conflict of interest relevant to this article was reported.