 |
 |
- Search
| Obstet Gynecol Sci > Volume 65(3); 2022 > Article |
|
Abstract
Objective
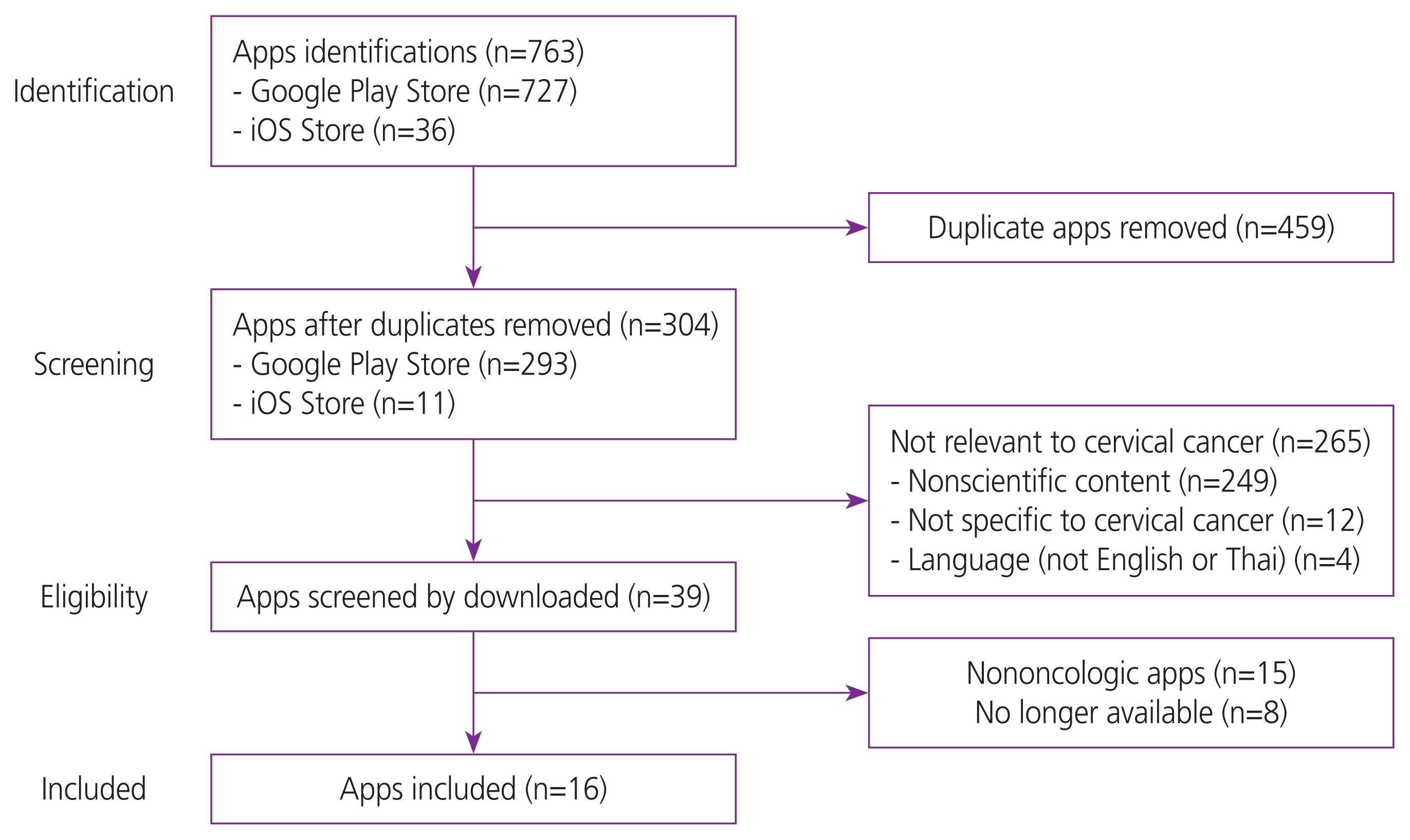
Methods
Results
Conclusion
Acknowledgments
Notes
Ethical approval
Our study was exempt from ethical approval by the Ethics Committee of Human Research, Khon Kaen University, as it did not involve human subjects.
Fig.┬Ā1
Table┬Ā1
| App name | Objective quality section | Objective quality score (5 points) | Subjective quality score (5 points) | Overall quality MARS score (5 points) | |||
|---|---|---|---|---|---|---|---|
| Engagement (5 points) | Functionality (5 points) | Aesthetics (5 points) | Information (5 points) | ||||
| 1. Mareng Pak Mdluka) | 2.20 | 4.00 | 2.33 | 3.29 | 2.95 | 2.75 | 2.85 |
| 2. Cervical cancer guide | 2.20 | 4.25 | 2.00 | 3.14 | 2.90 | 2.25 | 2.58 |
| 3. Cervical Cancer (Bedieman) | 1.60 | 3.75 | 1.00 | 3.29 | 2.41 | 2.25 | 2.33 |
| 4. Cervical Cancer (Nature Healthy Care) | 1.80 | 3.75 | 2.67 | 3.14 | 2.84 | 1.75 | 2.30 |
| 5. Cervical Cancer (Fumo) | 1.80 | 4.25 | 1.67 | 3.29 | 2.75 | 2.25 | 2.50 |
| 6. MyPap | 3.40 | 4.25 | 2.67 | 3.29 | 3.40 | 3.75 | 3.58 |
| 7. Scanvix | 2.40 | 3.00 | 2.33 | 2.85 | 2.65 | 1.25 | 1.95 |
| 8. Cervical Cancer Symptoms | 2.20 | 2.75 | 2.00 | 2.71 | 2.42 | 1.50 | 1.96 |
| 9. Cervical cancer (Anastore) | 2.40 | 2.00 | 1.00 | 2.71 | 2.03 | 1.00 | 1.52 |
| 10. TNM Cancer Staging Calculator | 3.00 | 4.25 | 3.67 | 3.43 | 3.59 | 3.25 | 3.42 |
| 11. BSCCP | 3.00 | 3.00 | 3.33 | 3.33 | 3.08 | 1.25 | 2.17 |
| 12. Cancer Genetics | 2.40 | 3.50 | 2.33 | 3.43 | 2.92 | 1.00 | 1.96 |
| 13. FIGO Gyn Cancer Management | 4.20 | 3.50 | 3.00 | 4.57 | 3.82 | 3.25 | 3.54 |
| 14. NCCN Guidelines® | 1.80 | 3.00 | 2.67 | 4.86 | 3.08 | 4.00 | 3.54 |
| 15. Cervical Cancer (Personal Remedies LLC) | 1.80 | 2.00 | 2.00 | 2.14 | 1.99 | 1.00 | 1.50 |
| 16. ASCCP Management Guidelines | 3.00 | 4.00 | 4.00 | 4.86 | 3.96 | 4.00 | 3.98 |
| Mean┬▒standard deviation | 2.45┬▒0.71 | 3.45┬▒0.76 | 2.42┬▒0.84 | 3.40┬▒0.76 | 2.92┬▒0.57 | 2.28┬▒1.10 | 2.61┬▒0.79 |
App, application; MARS, mobile app rating scale; TNM, tumor, nodes, and metastases; BSCCP, The British Society for Colposcopy and Cervical Pathology; FIGO, The International Federation of Gynecology and Obstetrics; NCCN, The National Comprehensive Cancer Network; LLC, Limited Liability Company; ASCCP, The American Society for Colposcopy and Cervical Pathology.
Table┬Ā2
| App name | APPLICATION scoring system | Total score (16 points) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | P | P | L | I | C | A | T | I | O | N | S | ||
| 1. Mareng Pak Mdluka) | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 9 |
| 2. Cervical cancer guide | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 11 |
| 3. Cervical Cancer (Bedieman) | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 9 |
| 4. Cervical Cancer (Nature Healthy Care) | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 7 |
| 5. Cervical Cancer (Fumo) | 3 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 8 |
| 6. MyPap | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 7 |
| 7. Scanvix | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 7 |
| 8. Cervical Cancer Symptoms | 3 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 10 |
| 9. Cervical cancer (Anastore) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 6 |
| 10. TNM Cancer Staging Calculator | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 8 |
| 11. BSCCP | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| 12. Cancer Genetics | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 6 |
| 13. FIGO Gyn Cancer Management | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 8 |
| 14. NCCN Guidelines® | 3 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 10 |
| 15. Cervical Cancer (Personal Remedies LLC) | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 8 |
| 16. ASCCP Management Guidelines | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 11 |
| Mean┬▒standard deviation | 1.94┬▒1.00 | 0.88┬▒0.34 | 0.94┬▒0.25 | 0.38┬▒0.50 | 0.81┬▒0.40 | 0.50┬▒0.52 | 0.63┬▒0.50 | 0.13┬▒0.34 | 0.44┬▒0.51 | 0.75┬▒0.68 | 0.75┬▒0.45 | 0.38┬▒0.50 | 8.50┬▒1.71 |
App, application; A, app comprehensiveness (3 points); P, price (1 point); P, paid subscription (1 point); L, literature used (1 point); I, in-app purchase (1 point); C, connectivity (1 point); A, advertisements (1 point); T, text search field (1 point); I, interdevice compatibility (1 point); O, other component (1 point); N, navigation ease (1 point); S, subjective presentation (1 point); TNM, tumor, nodes, and metastases; BSCCP, The British Society for Colposcopy and Cervical Pathology; FIGO, The International Federation of Gynecology and Obstetrics; NCCN, The National Comprehensive Cancer Network; LLC, Limited Liability Company; ASCCP, The American Society for Colposcopy and Cervical Pathology.
Table┬Ā3
1, Mareng Pak Mdluk; 2, Cervical cancer guide; 3, Cervical Cancer (Bedieman); 4, Cervical Cancer (Nature Healthy Care); 5, Cervical Cancer (Fumo); 6, MyPap; 7, Scanvix; 8, Cervical Cancer Symptoms; 9, Cervical cancer (Anastore); 10, TNM Cancer Staging Calculator; 11, BSCCP; 12, Cancer Genetics; 13, FIGO Gyn Cancer Management; 14, NCCN Guidelines®; 15, Cervical Cancer (Personal Remedies LLC); 16, ASCCP Management Guidelines.
App, application; HPV, human papillomavirus; TNM, tumor, nodes, and metastases; BSCCP, The British Society for Colposcopy and Cervical Pathology; FIGO, The International Federation of Gynecology and Obstetrics; NCCN, The National Comprehensive Cancer Network; LLC, Limited Liability Company; ASCCP, The American Society for Colposcopy and Cervical Pathology.
Table┬Ā4
| App name (developer) | Platform | Category | Language | Update datesa) | Price (USD) | Download | Average star rating |
|---|---|---|---|---|---|---|---|
| 1. Mareng Pak Mdlukb) (Kimmydroid) | Android | Health and fitness | TH/EN | 04/11/2020 | 0 | >1,000 | - |
| 2. Cervical cancer guide (free apps for everyone) | Android | Health and fitness | EN | 28/05/2020 | 0 | >5,000 | 3.8 |
| 3. Cervical Cancer (Bedieman) | Android | Health and fitness | EN | 11/04/2020 | 0 | >100 | - |
| 4. Cervical Cancer (Nature Healthy Care) | Android | Entertainment | EN | 20/06/2020 | 0 | >1,000 | - |
| 5. Cervical Cancer (Fumo) | Android | Medical | EN | 12/04/2020 | 0 | >1,000 | - |
| 6. MyPap (Ovidiu Miu) | Android | Health and fitness | EN | 06/02/2021 | 0 | >50 | - |
| 7. Scanvix (Charles Ingorot) | Android | Health and fitness | EN | 06/10/2019 | 0 | >100 | 4.8 |
| 8. Cervical Cancer Symptoms (Revolxa Inc.) | Android | Medical | EN | 01/03/2021 | 0 | >1,000 | 4.8 |
| 9. Cervical cancer (Anastore) | Android | Medical | EN | 22/03/2017 | 0 | >100 | 4.1 |
| 10. TNM Cancer Staging Calculator (Integrated Cancer Research) | Android and iOS | Medical | EN | 22/03/2018 | 0 | >10,000 | 4.5 |
| 11. BSCCP (Thursday Studio) | Android and iOS | Medical | EN | 22/07/2020 | 0 | >500 | - |
| 12. Cancer Genetics (UBQO Limited) | Android and iOS | Medical | EN | 18/02/2016 | 0 | >1,000 | - |
| 13. FIGO Gyn Cancer Management (International atomic energy agency) | Android and iOS | Health and fitness | EN | 09/04/2019 | 0 | >10,000 | 4.7 |
| 14. NCCN Guidelines® (NCCN) | Android and iOS | Medical | EN | 24/06/2020 | 0 | >100,000 | 3.0 |
| 15. Cervical Cancer (Personal Remedies LLC) | Android and iOS | Medical | EN | 02/12/2019 | 3.49 | >1 | - |
| 16. ASCCP Management Guidelines (ASCCP) | Android and iOS | Medical | EN | 14/10/2020 | 9.99 | >5,000 | 2.8 |
App, application; EN, English language; TH, Thai language; TNM, tumor, nodes, and metastases; BSCCP, The British Society for Colposcopy and Cervical Pathology; UBQO, The Specific Name of Private limited Company; FIGO, The International Federation of Gynecology and Obstetrics; NCCN, The National Comprehensive Cancer Network; LLC, Limited Liability Company; ASCCP, The American Society for Colposcopy and Cervical Pathology.
Table┬Ā5
| App name | Assessment tools | Average star rating | ||
|---|---|---|---|---|
| Total MARS score (5 points) | APPLICATIONS scoring system (16 points) | Apps rating using specific statements (16 points) | ||
| 1. Mareng Pak Mdluka) | 2.85 | 9 | 8 | 0 |
| 2. Cervical cancer guide | 2.58 | 11 | 11 | 3.8 |
| 3. Cervical Cancer (Bedieman) | 2.33 | 9 | 11 | 0 |
| 4. Cervical Cancer (Nature Healthy Care) | 2.30 | 7 | 12 | 0 |
| 5. Cervical Cancer (Fumo) | 2.50 | 8 | 11 | 0 |
| 6. MyPap | 3.58 | 7 | 1 | 0 |
| 7. Scanvix | 1.95 | 7 | 8 | 4.8 |
| 8. Cervical Cancer Symptoms | 1.96 | 10 | 10 | 4.8 |
| 9. Cervical cancer (Anastore) | 1.52 | 6 | 14 | 4.1 |
| 10. TNM Cancer Staging Calculator | 3.42 | 8 | 0 | 4.5 |
| 11. BSCCP | 2.17 | 11 | 3 | 0 |
| 12. Cancer Genetics | 1.96 | 6 | 7 | 0 |
| 13. FIGO Gyn Cancer Management | 3.54 | 8 | 0 | 4.7 |
| 14. NCCN Guidelines® | 3.54 | 10 | 13 | 3.0 |
| 15. Cervical Cancer (Personal Remedies LLC) | 1.50 | 8 | 7 | 0 |
| 16. ASCCP Management Guidelines | 3.98 | 11 | 9 | 2.8 |
| Mean┬▒standard deviation | 2.60┬▒0.79 | 8.50┬▒1.71 | 7.81┬▒4.56 | - |
App, application; MARS, mobile app rating scale; TNM, tumor, nodes, and metastases; BSCCP, The British Society for Colposcopy and Cervical Pathology; FIGO, The International Federation of Gynecology and Obstetrics; NCCN, The National Comprehensive Cancer Network; LLC, Limited Liability Company; ASCCP, The American Society for Colposcopy and Cervical Pathology.
References
- TOOLS
-
METRICS

- Related articles in Obstet Gynecol Sci
-
Evaluation of obesity as a potential risk factor for cervical cancer.2004 December;47(12)




















