Beneficial effects of Teucrium polium hydroalcoholic extract on letrozole-induced polycystic ovary syndrome in rat model
Article information
Abstract
Objective
Polycystic ovary syndrome (PCOS) is an endocrine disorder that disrupts the menstrual cycle and causes infertility. Considering the increasing use of medicinal plants, the present study aimed to evaluate the effects of Teucrium polium L. on letrozole-induced PCOS in female rats.
Methods
Six groups of rats (n=7 each) were evaluated. The control group received 1% carboxy methyl cellulose as vehicle, while the five other groups received letrozole 1 mg/kg orally for 21 days. After PCOS induction, the rats were orally administered T. polium extract (50, 100, and 200 mg/kg) or metformin (200 mg/kg) for 28 days. Subsequently, body and ovarian weights and serum levels of follicle stimulating hormone, luteinizing hormone (LH), estradiol, progesterone, and testosterone were measured. Finally, the ovarian tissues were isolated for histological examination.
Results
There were no significant changes in weekly body weight in any group. After 21 days of letrozole administration, PCOS induction was confirmed by estrous cycle irregularities and increased LH and testosterone levels. After treatment with the hydroalcoholic extract of T. polium, testosterone and LH levels were significantly reduced in all groups (P<0.05). Histological studies of ovaries in the metformin and T. polium groups exhibited normal follicular development with fewer and smaller cystic follicles than those in the PCOS group.
Conclusion
The hydroalcoholic extract of T. polium improves serum levels of sex hormones, restores ovarian morphology in PCOS-induced rats, and is a good candidate for further clinical trials.
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine disease with a 5–10% worldwide incidence in women [1]. Prolonged or irregular menstruation and hyperandrogenism have been observed in affected women. In such cases, a woman’s chances of pregnancy decrease. Acne, oily skin, excess facial and body hair growth, and hair loss are the symptoms of PCOS [2]. Metabolic abnormalities such as diabetes, dyslipidemia, coronary heart disease, and gynecological cancer have also been observed in PCOS [3].
Persistent hyperandrogenism is linked to insufficient hypothalamic-pituitary feedback, luteinizing hormone (LH) hypersecretion, and arrested antral follicle formation [4]. In PCOS, estrogen and progesterone levels are decreased; however, testosterone levels are increased, and the LH/follicle stimulating hormone (LH/FSH) ratio becomes three times higher than normal [1].
Teucrium polium L., also known as felty germander (Kalpooreh in Persian), belongs to the lamiaceae family and includes more than 300 species. It is native to European countries, North Africa, and western Mediterranean areas, including Iran [5]. T. polium contains phytosterols (beta-sitosterol, stigmasterol, campesterol, brassicasterol, and clerosterol), flavonoids (apigenin-7-O-glucoside and cirsiliol), phenolic acids, and terpenoids [6,7].
T. polium has shown some biological activities such as diuretic, tonic, antipyretic, analgesic, antifungal, antibacterial, antispasmodic as well as anti-rheumatic effects. Furthermore, a study by Vahidi et al. [8] showed that T. polium has hypoglycemic effects. Salimnejad et al. [9] found that the alcoholic extract of T. polium alleviates the destructive changes associated with diabetes in murine testicles. Esmaeili et al. [10] showed that the flavonoids of T. polium extract (rutin and apigenin) protect the islets of Langerhans in the pancreas against destructive effects owing to their antioxidant activity. Abadian et al. [11] found that T. polium significantly reduces the duration and amount of menstrual bleeding in 2 consecutive weeks of menstruation cycles. Another study revealed that the hydroalcoholic extract of T. polium has a protective effect against diabetes-induced testicular damage, regulation of androgen receptors, and serum testosterone concentration. Because of the data reported in traditional studies, T. polium has been used as a diuretic, diaphoretic, tonic, emmenagogue, cholagogic, and a treatment of uterine infections [12,13].
Considering the increasing use of herbal medicine for the treatment of numerous diseases, such as female reproductive disorders [14], we aimed to investigate the effects of the hydroalcoholic extract of T. polium on ovarian tissue damage as well as hormonal aspects altered by PCOS in a letrozole-induced rat model of PCOS.
Materials and methods
1. Plant material and extract
Aerial parts of T. polium were collected from the Siahrood area in East Azerbaijan province of Iran and identified at the Herbarium of Traditional Medicine and Material Medicine Research Center (TMRC), Shahid Beheshti University of Medical Sciences, Tehran, Iran. A voucher specimen of Teucrium polium L. (no. 4513) was deposited at the Herbarium of TMRC.
Briefly, dried aerial parts of T. polium (200 g) were extracted with 3 L of ethanol (70% v/v) for 24 hours using the maceration method and then filtered. To obtain T. polium powder, the filtrate was concentrated and lyophilized using a freeze-dryer (Christ Alpha 2–4 LDplus; Martin Christ, Osterode am Harz, Germany). The final yield of T. polium extract was 13.50% w/w.
2. Reagents
Analytical grade chemicals and reagents included letrozole (Tofigh Daru Pharmaceutical Co., Tehran, Iran), powdered metformin (Mahban Chemi Co., Tehran, Iran), carboxymethyl cellulose (CMC; Merck, Darmstadt, Germany), and Hematoxylin and Eosin stain (Padtanteb, Tehran, Iran). Serum 17β-estradiol (E2) and testosterone levels were calculated using an enzyme-linked immunosorbent assay (ELISA) kit (KGE014; R&D Systems, Minneapolis, MN, USA). Serum progesterone levels were calculated using a mouse/rat progesterone ELISA kit (catalog number SE120087; Sigma-Aldrich, Darmstadt, Germany). Serum LH levels were evaluated using an ELISA kit (catalog number MBS 729873; MyBioSource, San Diego, CA, USA), and FSH levels were evaluated using an ELISA kit (catalog MBS 2502190 from MyBioSource, San Diego, CA, USA).
3. Animals and treatment
Adult female Sprague-Dawley rats (6–8 weeks old; mean weight, 120 g) were bred in the animal house of the TMRC. All animals were kept in a well-ventilated animal facility in temperature-controlled rooms (22°C) with 45–65% humidity and a 12-hour light and dark cycle. Water and a standard diet were provided ad libitum. Their body weights were monitored weekly. The experimental protocol was approved by the Experimental Animal Ethics Committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH. REC.1400.230).
A practical model of the experiment is shown in Fig. 1. Vaginal smears were obtained daily from each rat, and those with two consecutive regular estrous cycles were included in the study. The animals were randomly divided into six groups (n=7 each). The first group served as the control group and received 1 mL of 1% CMC (vehicle). The other groups were administered letrozole 1 mg/kg p.o. dissolved in 1% CMC (2 mL/kg) once daily for 21 days. This model was chosen based on previous studies in which cystic follicle formation was induced [15]. Vaginal smears were collected daily to verify the induction of PCOS. One rat from each group was sacrificed 21 days after letrozole treatment. Biochemical and histological tests were performed to confirm PCOS in the rats. From day 22, the animals in the second group were gavaged with distilled water (PCOS group), those in the third to fifth groups were gavaged with T. polium hydroalcoholic extract at doses of 50, 100, and 200 mg/kg (T. polium groups), and the animals in the sixth group were treated with metformin (metformin group) 200 mg/kg [16]. The test materials were administered for 28 days.
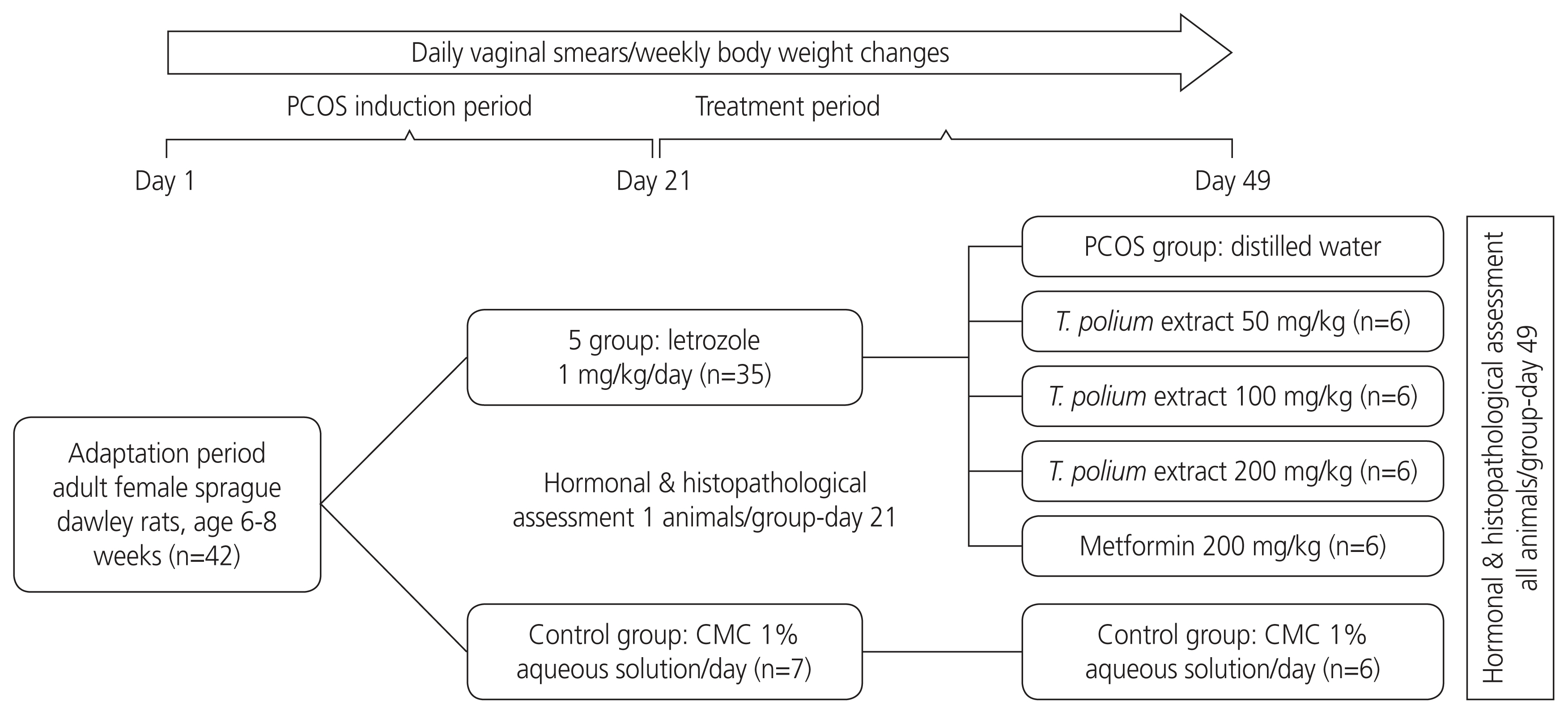
The experimental model of the study. PCOS, polycystic ovarian syndrome; CMC, carboxymethyl cellulose.
After the treatment period, all rats were anesthetized with ketamine/xylazine (5/1 mg/kg) [17]. Blood samples were collected from the inferior vena cava of all rats, and the serum was separated by centrifugation for 10 minutes at 2,500 rpm.
4. Hormonal assay
The levels of LH, FSH, estrogen, progesterone, and testosterone were measured in the serum collected from the rats using a rat ELISA kit.
5. Histopathological studies
Ovary samples were removed from the rats immediately after the last blood collection. The weight of each ovary was measured and the ovaries from each rat were fixed in 10% neutral-buffered formalin for 48 hours. Subsequently, the tissues were embedded in paraffin and cut into sections (5 μm thick). The ovaries were stained with hematoxylin and eosin according to standard histological procedures. Pathological-physiological structures in the ovaries were examined under a light microscope (Ceti Microscopes, Chalgrove, Warpsgrove, UK).
6. Statistical analysis
The statistical analysis was performed using GraphPad Prism version 9.00 (GraphPad, San Diego, CA, USA). The results are expressed as mean±standard error of the mean. One-way analysis of variance (ANOVA) with the Tukey-Kramer multiple pair comparison test and two-way ANOVA with the Bonferroni post hoc test were used to determine significant differences between groups. Statistical significance was set at P<0.05.
Results
1. Animal weights
A slow increase in body weight was observed in all four groups. Two-way ANOVA with repeated measures using day and group as variables showed no significant change in weekly body weight in all groups (Fig. 2A). There were insignificant differences in mean ovarian weights among the treatment, control, and PCOS groups (Fig. 2B).
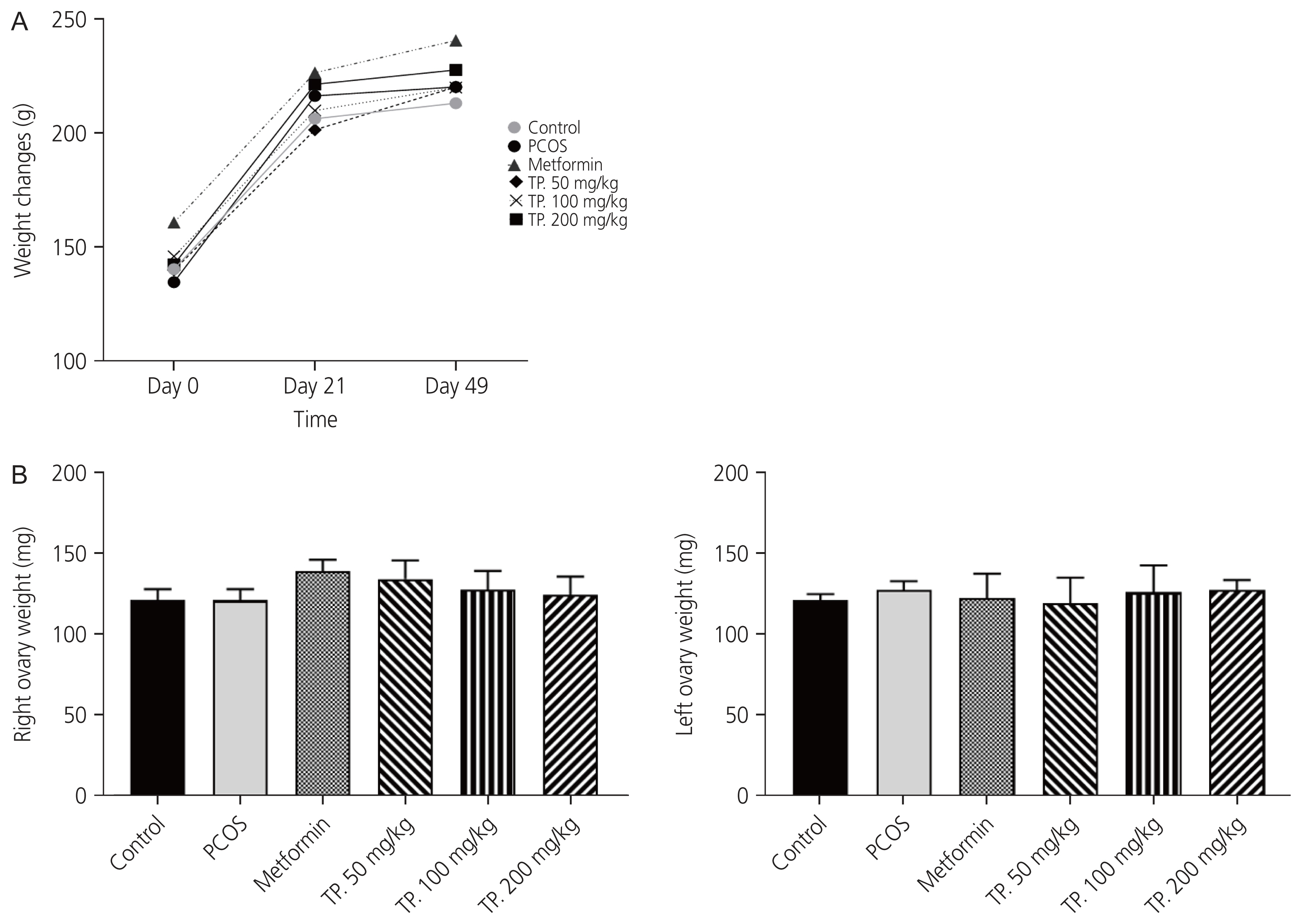
(A) Bodyweight over time. Each point represents the average body weight (in grams) for each group, and the trend over three-time points is shown. Two-way ANOVA with repeated measures using the day and group as variables showed no significant change between groups. (B) Bilateral ovaries’ weights measurement. Analyzed by one-way ANOVA test with Tukey’s compare all pair of columns. Data are expressed as mean±standard error of the mean. PCOS, polycystic ovarian syndrome; TP., Teucrium polium; ANOVA, analysis of variance.
2. Estrus cyclicity after treatment
All rats in the control group had a regular estrus cycle, while all untreated PCOS rats had an irregular estrus cycle for 28 days. The hydroalcoholic extracts of T. polium and metformin increased the percentage of rats with regular estrus cyclicity versus the PCOS group. These substantial increases peaked in the fourth week in all groups and had the highest percentage of T. polium extracts (100 mg/kg and 200 mg/kg) (Table 1).
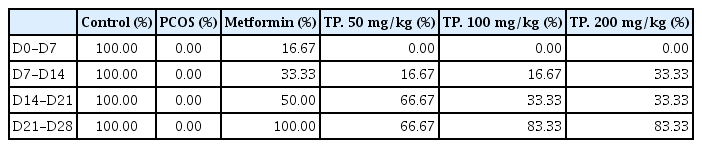
Percentage of PCOS rats with regular estrus cycle after treatment with metformin or hydroalcoholic extracts of TP. for 28 days
The percentage of different phases of the estrus cycle after 28 days of drug and metformin administration was similar to that of the control group, indicating that the estrus cycle became more regular in these groups. Consequently, the percentage of the diestrus phase was significantly lower in the metformin and T. polium extract groups than in the PCOS group. In addition, the percentage of the estrus phase, which significantly decreased after PCOS induction, was significantly higher in the metformin and T. polium extract groups than in the PCOS group. The percentage of the proestrus phase also increased significantly in the 50 and 200 mg/kg T. polium extract groups versus that in the PCOS group (Fig. 3).
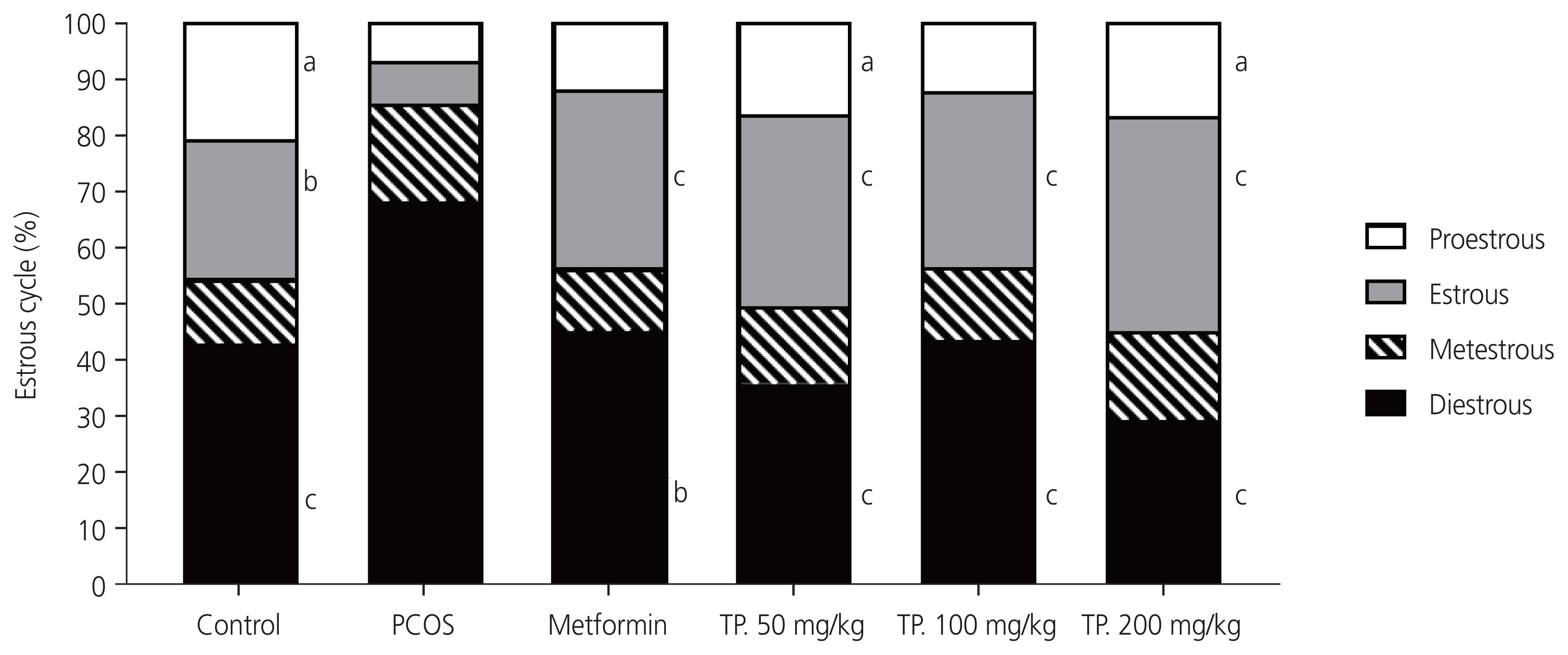
Percentage of each phase of the estrus cycle after treatment with metformin and hydroalcoholic extracts of Teucrium polium for 28 days. Number of rats per group, 6. Values are expressed as mean±standard error of the mean (n=6) evaluated by one-way analysis of variance followed by Tukey’s test. (a) P<0.05; (b) P<0.001; (c) P<0.0001 versus the PCOS group. PCOS, polycystic ovarian syndrome; TP., Teucrium polium.
3. Serum hormonal analysis
Compared to the control group, letrozole administration significantly increased serum LH levels (P<0.001; Fig. 4A). Treatment with T. polium extract and metformin reversed the letrozole effect and significantly decreased LH levels versus those in PCOS rats.

Mean±standard error of the mean of LH, FSH, testosterone, progesterone, and estradiol concentrations in control, PCOS, metformin, and TP. hydroalcoholic extracts groups after 28 days of the experiment. (A) LH, (B) FSH, (C) LH/FSH ratio, (D) estradiol, (E) testosterone, and (F) progesterone. Values are expressed as mean±standard error of the mean (n=6). As evaluated by one-way ANOVA followed by Tukey’s tests. LH, luteinizing hormone; PCOS, polycystic ovarian syndrome; TP., Teucrium polium; FSH, follicle stimulating hormone; ANOVA, analysis of variance. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 compared to the PCOS group. ##P<0.01; ###P<0.001; ####P<0.0001 as compared to the control group.
The administration of letrozole did not result in any significant changes in the plasma levels of FSH in the PCOS versus control group (Fig. 4B). Similarly, treatment with T. polium extracts (50, 100, and 200 mg/kg) and metformin did not significantly change FSH levels compared to those of the PCOS rats.
FSH and LH levels and their ratio play crucial roles in ovulation. A key symptom of PCOS is a 2- to 3-fold increase in the LH/FSH ratio. The LH/FSH ratio was significantly increased in the PCOS group but effectively regulated in the metformin and T. polium extract groups. This downregulation was significant only in the T. polium extract 50 mg/kg group (P<0.05) (Fig. 4C).
The E2 levels were significantly decreased (P<0.01) in PCOS versus control rats. Treatment with the T. polium extract at 200 mg/kg (P<0.01) and metformin (P<0.0001) resulted in a significant increase in E2 levels compared to that in PCOS rats (Fig. 4D).
Progesterone levels were significantly reduced (P<0.001) in the PCOS group compared to those in the control group (Fig. 4E). Treatment with T. polium extract (50 mg/kg) and metformin significantly elevated progesterone levels (P<0.05, and P<0.0001, respectively) in comparison to the PCOS rats.
The testosterone level in the PCOS group was significantly higher than that in the control group (P<0.0001) on the final day of the study (Fig. 4F). Treatment with 50 and 100 mg/kg of the extracts (P<0.05, P<0.01) and metformin (P<0.0001) showed a significant decrease in testosterone levels compared to PCOS rats.
4. Histopathological studies of ovaries
The control group showed normal histological features of ovaries (Fig. 5A). The PCOS group displayed many large ovarian cystic follicles, attenuation of the granulosa cell layer, and a few corpora lutea (Fig. 5B). Rats treated with T. polium extract and metformin exhibited ovarian tissue with well-developed antral and graafian follicles, along with corpora lutea and a normal granulosa cell layer (Fig. 5C–F). Ovarian cystic follicles were significantly reduced in all the extract and metformin groups compared to those in the PCOS group. The group treated with 50 mg/kg extract exhibited ovarian tissue with a significant increase in graafian follicles (P<0.01) and corpora lutea (P<0.01) compared with the PCOS group (Table 2).
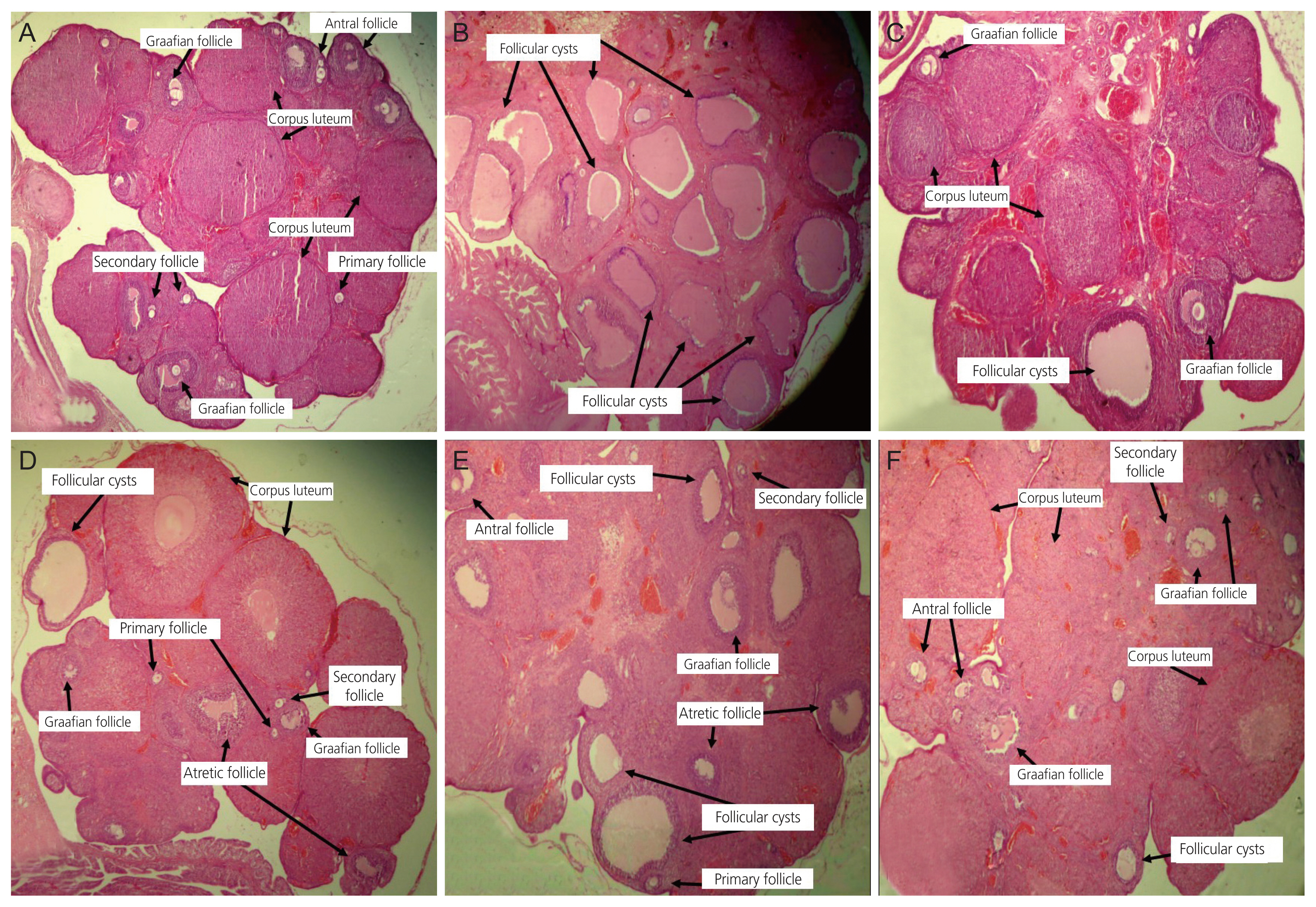
Microscopic study of lateral section of rat ovary. Histological sections of the ovary were stained with H&E, ×4. Treated rats after 28 days of the experiment. (A) Control group with different stages of ovarian follicles including secondary, antral, graafian follicles, and corpora lutea. (B) PCOS group containing various giant cystic follicles. (C) Metformin group with secondary, antral, graafian follicles, and corpora lutea. (D) TP. 50 mg/kg group with few cystic follicles and other follicles. (E) TP. 100 mg/kg group with mature graafian follicles, few cystic follicles, and other developing follicles and corpora lutea. (F) TP. 200 mg/kg group with lots of graafian and antral follicles and small cystic follicles. H&E, Hematoxylin and Eosin stain; PCOS, polycystic ovarian syndrome; TP., Teucrium polium.
Discussion
PCOS is a hormonal disorder that is very common in women of childbearing age. PCOS features symptoms that affect the ovaries and ovulatory process [18]. Women with PCOS have higher than normal androgen levels, which is known as hyperandrogenism. This hormonal imbalance disrupts the menstrual cycle and fertility. In this syndrome, the ovaries may produce small fluid-filled cysts (follicles/follicles) and regularly show impaired ovulation [19]. In the present study, we investigated the effects of oral T. polium extract on the biochemical and histological features of PCOS in a rat model.
Since human studies have many limitations, numerous animal models that mimic many or all PCOS features have been developed [1]. Polycystic ovaries can be induced by excess secondary endogenous androgen [20]. This can be achieved using letrozole, an aromatase inhibitor, which blocks the conversion of androgens to estrogen [19]. A decrease in aromatase activity, which is typically expressed in the ovary, could increase the intraovarian production of androgens, which simultaneously decreases estrogen levels, leading to the development of polycystic ovaries. The oral administration of letrozole to adult rats for at least 21 consecutive days induces acyclicity [21] and irregular estrus cycles [1]. Endocrine disturbances, including elevated LH and testosterone levels and decreased FSH levels reflecting the accumulation of endogenous ovarian androgen secretion, can be seen in this animal model, mimicking human PCOS. Decreased progesterone secretion after PCOS induction has been observed in studies using this animal model [1]. As anticipated in the current study, letrozole efficiently induced PCOS in rats after 21 days of an oral gavage. PCOS induction was confirmed by irregular estrous cycles and increased testosterone and LH levels.
The estrous cycle becomes irregular in rats with PCOS, primarily due to altered levels of the sex hormones that regulate ovarian function [22]. In our study, PCOS rats had irregular estrus cyclicity, whereas control rats showed regular cyclicity after 21 days of vehicle treatment. Similarly, two other studies documented that letrozole-induced PCOS is associated with a prolonged estrus cycle in female rats [23,24]. The treatment of PCOS rats with metformin and hydroalcoholic extract of T. polium conceivably corrected the estrous cyclicity by modulating the aromatization of androgens into estrogen by lowering LH levels, improving circulating E2 concentrations, and inducing ovulation.
Measurements of sex hormone levels (particularly testosterone, LH, and E2) is used for diagnosing PCOS [15]. Indeed, elevated serum testosterone and LH concentrations and low E2, progesterone, and FSH levels are the most consistent hormonal features for diagnosing PCOS in women [25]. In this study, the PCOS group showed high LH and testosterone levels but low E2 and progesterone concentrations compared to the control group. These outcomes are consistent with previous results reported by other researchers [26,27]. Treatment by T. polium improved hyperandrogenism in rats as evidenced by significantly decreased testosterone and LH levels after 28 days of oral treatment, which could promote follicular development and cause ovulation. Among the three different doses of T. polium, the 50 mg/kg dose showed the most beneficial effect on these hormonal modifications. T. polium contains β-sitosterol, campesterol, and stigmasterol, which have antiandrogenic properties that reduce testosterone levels by inhibiting the dihydrotestosterone-receptor complex [28–30]. In contrast, Al-Tikriti et al. [31] reported that the hexane extract of T. polium caused a significant increase in testosterone levels in male rats owing to the downregulation of androgen receptors. Since an abnormal increase in testosterone levels contributes to the pathogenesis of PCOS [19], its downregulation after T. polium treatment may have beneficial effects on reproductive disorders related to PCOS.
The long-term use of plant extracts containing phytoestrogens, such as T. polium, can reduce testosterone levels with negative feedback to LH [32]. Therefore, LH is probably produced to a lesser extent following a decrease in androgen levels, and the dominant effect of LH on FSH is reduced. In our study, LH levels significantly decreased after the administration of T. polium. The LH/FSH ratio is typically 1:1; however, this ratio is 2 or 3 times higher in PCOS cases [33]. A recent study showed that normalizing the LH/FSH ratio is essential for PCOS treatment [34].
Our results showed that the LH/FSH ratio in PCOS rats was 2.3 times higher than that of the control group. This ratio normalized after T. polium extract administration. Similar to previous studies, FSH levels did not significantly change in our study [35,36]. A decrease in LH levels reduces the LH/FSH ratio. The improvement in LH, testosterone, E2, and progesterone levels by metformin was verified in previous studies. Metformin improves ovarian-related markers and induces ovulation in rats with PCOS [15,37].
Natural molecules, such as flavonoids, act on various pathological aspects of PCOS, including ovarian functionality, hormonal and metabolic profiles, inflammatory states, and oxidative stress. The mechanism of action of flavonoids has been investigated in several studies [38]. Ke and Duan [39] showed that flavonoids increase estrogen receptor expression in the hippocampus, hypothalamus, and pituitary glands as well as LH receptor expression in the ovaries. Rutin is a citrus flavonoid that has positive effects on PCOS. Rutin is structurally similar to endogenous estrogen, which enables its absorption by target cells, binding to the estrogen receptor, and exertion of estrogen-like effects. It also improved the plasma and mammary concentrations of E2 in ovariectomized virgin rats and upregulated the expressions of estrogen and growth hormone receptors [40]. Another flavonoid, apigenin, ameliorates the disturbed hormonal levels, lipid profile, and antioxidant status in PCOS rats [41].
Since T. polium also contains considerable flavonoid compounds, such as rutin and apigenin, these compounds may be responsible for the beneficial effects on PCOS through the aforementioned mechanisms.
Histology is a prerequisite for recognizing ovarian changes. Our results showed that the ovaries of the PCOS group had multiple enlarged cysts lacking an oocyte, granulosa layer hyperplasia, and increased follicular atresia consistent with the findings of previous studies [19,42]. The decreased number of antral and graafian follicles reflected androgen overproduction, which restricted the normal follicular maturation process in the PCOS group [42]. In contrast, the metformin and T. polium groups demonstrated remarkable recovery of the ovarian tissue and the appearance of developing and antral follicles, a noticeable reduction in cysts, and regular luteinization. The presence of more corpora lutea in the metformin and T. polium groups indicated the return of the estrus cycle to normal [42]. The destructive effects of letrozole on ovarian tissue were corrected by T. polium extract, probably owing to its antioxidant and antiandrogenic properties. Many studies have investigated the antioxidant- and free radical-scavenging properties of T. polium using in vitro models. Antioxidant enzyme levels reportedly decline in patients with PCOS [43].
Our experimental results suggest that the hydroalcoholic extract of T. polium improves ovarian function in rats with letrozole-induced PCOS. T. polium has beneficial effects on hormonal indices, estrus cyclicity, and ovarian histology. The T. polium 50 and 100 mg/kg doses were effective, and our findings suggest that doses of less than 50 mg/kg should be investigated in future studies to determine the most effective dose. Owing to the phytoestrogen content of T. polium and its ability to rehabilitate ovarian function, it may offer an advantageous remedy for PCOS. Likewise, clinical studies are needed to explore the therapeutic potential so that T. polium can be used as an adjunct therapy for PCOS.
Acknowledgments
The authors appreciate the participants of this study. This article was extracted from Miss Khosrowpour’s thesis for a Ph.D. degree (225) at Shahid Beheshti University of Medical Sciences. This study was financially supported by the School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (no. 00-27857). The letrozole and metformin powders were obtained from Tofigh Daru Pharmaceutical Co. and Mahban Chemi Co., Iran.
Notes
Conflict of interest
None.
Ethical approval
The Ethics Committee of Shahid Beheshti University of Medical Sciences approved the proposal of the research. The approval code number is (IR.SBMU.RETECH.REC.1400.230).
Patient consent
None.
Funding information
The project was granted by Traditional Medicine and Materia Medica Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The institute had no role in the design of the study, collection, analysis, and interpretation of data, or writing the manuscript.

