|
 |
- Search
| Obstet Gynecol Sci > Volume 66(3); 2023 > Article |
|
Abstract
Objective
To assess the correlation between the intention to undergo immediate versus delayed postpartum contraceptive implant insertion following high-risk pregnancy, and the proportion of utilization and adverse effects.
Methods
We conducted a retrospective cohort study of women who gave birth after a high-risk pregnancy (according to the criteria defined by the Society for Maternal-Fetal Medicine) and intended to use contraceptive implants. The participants were classified into two groups based on whether they underwent immediate or delayed insertion. The primary outcome was the proportion of utilization of contraceptive implants at 12 months postpartum. We performed multivariate analyses to determine the relationships between the timing of insertion, characteristics, and methods used.
Results
Of the 482 women classified as having high-risk pregnancies, 103 intended to use contraceptive implants (54 immediate and 49 delayed insertions). Women in the immediate group were more likely to use contraceptive implants than those in the delayed group at 6 (95.2% vs. 26.2%, P<0.01) and 12 months (92.7% vs. 26.2, P<0.01). A higher proportion of participants in the immediate group reported spotting and prolonged bleeding at 12 months (51.1% vs. 23.8%, P=0.01 and 26.8% vs. 7.1%, P=0.01; respectively). However, satisfaction at 12 months was higher in the immediate group than in the delayed group.
Conclusion
Intention to undergo implant insertion during the immediate postpartum period appears to improve the utilization of highly effective contraception. Patients who underwent immediate implantation experienced more spotting, prolonged bleeding, and dysmenorrhea. This study supports the recommendation to provide immediate postpartum contraceptive implants to women following high-risk pregnancies.
High-risk pregnancy is defined by the Society for Maternal-Fetal Medicine (SMFM) as a pregnancy that poses a significant risk of mortality or morbidity to the mother, fetus, or newborn. This risk is due to maternal or fetal health issues as well as non-medical contextual factors that demand more resources or interventions to enhance outcomes [1]. Additionally, women with medical comorbidities are more vulnerable to unintended pregnancy than those without [2]. The combination of pregnancy and uncontrolled underlying disease is linked to a greater risk of maternal and fetal morbidities and disease progression [3].
Optimizing the interpregnancy interval is imperative for women at high risk of maternal/fetal morbidity and mortality. Short inter-pregnancy interval are associated with adverse maternal and infant health outcomes, even in healthy populations [4]. Therefore, the SMFM recommends that obstetric care providers offer highly effective contraception to these women, particularly immediate postpartum (IPP) insertion of long-acting reversible contraception (LARC) [3]. Contraceptive implants are a form of LARC that prevent pregnancy over a 3 to 5-year period, depending on the type of implant, by inhibiting ovulation and modifying the endometrial lining and cervical mucosa. The typical failure rate of this method is the lowest among all types of LARC [5]. Although there are some concerns regarding interaction between progestin in the contraceptive implant and underlying diseases, most women are eligible for the procedure and it is regarded as a safe method [5,6].
In our previous study, contraceptive implants were well accepted by postpartum women following high-risk pregnancies; the uptake rate was approximately 10% [7]. However, a systematic review showed that the proportion of utilization in healthy women opting for immediate postpartum implants at 6 and 12 months were comparable to that of those opting for delayed insertion [8]. Furthermore, there is limited evidence regarding these differences among women with high-risk pregnancies. We hypothesized that high-risk women who express an intention to use IPP contraceptive implants are more likely to use this method at 12 months than those who intend to delay insertion. This study aimed to assess the impact of intention regarding the timing of contraceptive implant insertion on the proportion of utilization and side effects in this population.
This study was conducted using data of high-risk postpartum women who intended to use contraceptive implants between April and December 2020, from records obtained from Srinagarind Hospital, a university hospital in northeast Thailand. An investigator (TW) and a research assistant assessed the records of these women in December 2021 to ensure at least 1 year of usage. The Khon Kaen University Ethics Committee for Human Research approved this study (HE651105), which was registered with Thai clinical trials (TCTR20220314001). We have reported the data according to the Strengthening the Reporting of Observational Studies in Epidemiology statement [9].
Pregnancy complicated by medical or obstetric conditions is classified as high-risk according to the SMFM guidelines [1,3]. Medical conditions include chronic or infectious diseases which were pre-existing or developed during pregnancy. Obstetric conditions include fetal and maternal conditions linked to a higher risk of adverse outcomes.
Two types of contraceptive implants were available at our institute including one-rod 3-year etonogestrel implant (Implanon ®, MSD, Rahway, NJ, USA), and the two-rod 5-year LNG (Jadelle®, Schering OY, Turku, Finland). We did not provide antenatal counseling regarding contraception; therefore, this was performed after delivery. After receiving comprehensive postpartum counseling, women who intended to use contraceptive implants before discharge were categorized as the “immediate” group, while those who intended to undergo implantation at a postpartum visit were categorized as the “delayed” group. Normally, women are discharged from the hospital within 3 days after delivery and scheduled for follow-up visits within 12 weeks postpartum. Subsequently, both groups were scheduled for follow-up visits, on-site or via phone call, at 6 and 12 months postpartum.
We collected data on baseline characteristics, including age, parity, route of delivery, education level, family income, intention to become pregnant in the index pregnancy, previous contraception, intention to breastfeed, maternal medical conditions, and obstetric complications.
We monitored the records of these high-risk women for 12 months after delivery. The time points of interest were three, 6 and 12 months postpartum. Women with contraceptive implants at the time of follow-up were classified as “utilizing” and those without as “non-utilizing”. We also collected data on breastfeeding, reasons for early discontinuation of contraceptive implants, and whether patients in the non-utilizing group used other contraceptive methods. We categorized contraceptive methods into three tiers based on their effectiveness with typical use according to World Health Organization definitions [10]: tier 1 (permanent contraception, contraceptive implant, intrauterine device), tier 2 (depot medroxyprogesterone acetate, combined oral contraceptive pills, progestin-only pills), and tier 3 (condoms, withdrawal, and fertility awareness). We considered the tier 1 and tier 2 methods to be “highly effective”. Those who did not employ any methods were classified as “non-use”. We then compared the proportion of utilization of the “any” and “highly effective” methods in both the immediate and delayed group at three, 6 and 12 months. We used the criteria for bleeding patterns associated with contraceptive implants recently suggested by Creinin et al. [11].
Based on the assumption that the difference in the continuation of contraceptive implants between the immediate and delayed groups would be 29%, this study required a sample size of n=31 per group to provide 80% power and alpha 0.05 level comparison [12]. Assuming a 20% loss to follow-up, the final sample size was 49 patients per group. The primary outcome was the proportion of contraceptive implant utilization at 12 months in women with a prior high-risk pregnancy. Analyses were performed using SPSS Software version 16.0 (SPSS Inc., Chicago, IL, USA). We reported descriptive data using percentage, mean±standard deviation, or median and interquartile range. The baseline characteristics of the immediate and delayed insertion group were compared using a χ2 test or Fisher’s exact test and t-test, as appropriate. Because the loss-to-follow-up rate did not exceed the unacceptable level of the a priori target, we performed a complete case analysis. Univariate analysis by binary logistic regression was used to examine the relationships between demographic data and the use of contraceptive implants or other highly effective methods 12 months after delivery. Covariates with P values ≤0.20 in the univariate analysis were subjected to multivariable, backward stepwise logistic regression modelling.
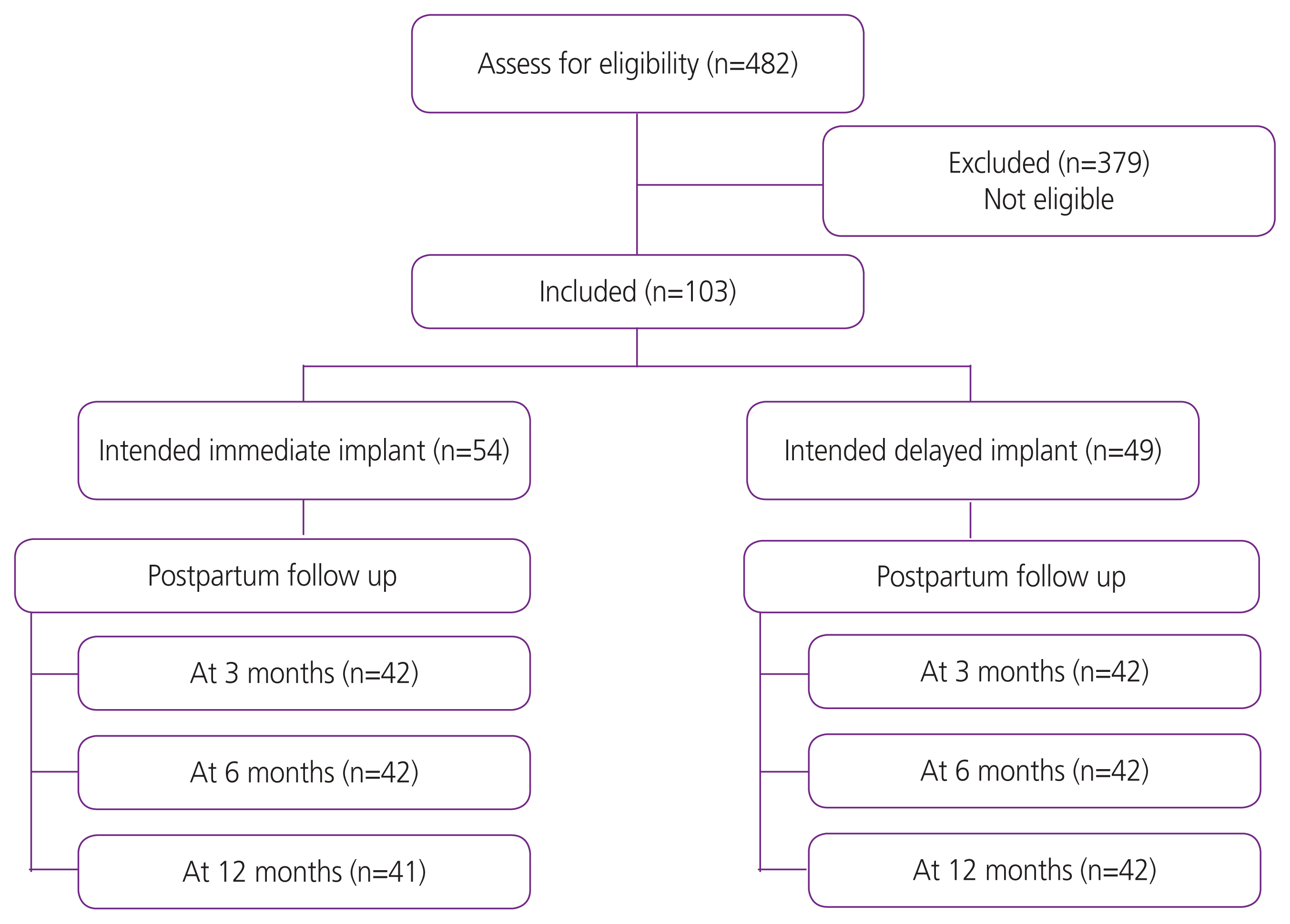
During the study period, 482 women were classified as having a high-risk pregnancy (Fig. 1). Of these, 103 (21.4%) intended to use contraceptive implants (54 immediate and 49 delayed insertion). The participant’s baseline characteristics and risk conditions in the immediate and delayed insertion groups are compared in Table 1. Women in the immediate group were slightly younger than those in the delayed group (27.3±6.8 vs. 30.2±5.9) and more likely to have become pregnant unintentionally (28.6% vs. 8.5%).
At 3 months, 84 women (42 in each group) returned to the hospital. Of these, 41/42 in the immediate intervention group had contraceptive implants inserted, whereas only 11/42 in the delayed intervention group were using the devices. The use of contraceptive implants at 6 and 12 months by the timing of intention to use is presented in Table 2. Women in the immediate group were more likely to use contraceptive implants than those in the delayed group at 6 and 12 months post-partum (95.2% vs. 26.2%, P<0.01, and 92.7% vs. 26.2, P<0.01; respectively). The proportion of women using any type of contraception was significantly higher in the immediate group during the first 6 months but did not differ at 12 months. A higher proportion of women in the immediate intervention group were using highly effective contraception than those in the delayed intervention group. However, a higher rate of side effects, including spotting, prolonged bleeding, and dysmenorrhea, was reported in the immediate intervention group at 12 months (51.2% vs. 23.8%, P=0.01, 26.8% vs. 7.1%, P=0.01, and 10.3% vs. 0%, P=0.03; respectively). Incidence of other side effects did not differ significantly between the groups (Table 3). However, satisfaction at 12 months remained higher in the immediate group than in the delayed group. None of the women in the delayed group had their devices removed prematurely compared to three in the immediate group. Reasons for early discontinuation included bleeding, headache, and hysterectomy for leiomyomas. None of the women became pregnant during the follow-up.
The proportion of women who breastfed in the immediate and delayed intervention groups were comparable at each time point. Furthermore, no significant differences in median breastfeeding satisfaction scores were observed between the two groups.
In our adjusted analysis, intention to use IPP contraceptive implant (adjusted odds ratio [OR] 67.64, 95% confidence interval [CI] 13.3-343.5) and delivery by cesarean section (adjusted OR 6.50, 95% CI 1.51-28.07) were associated with implant utilization at 12 months (Table 4). Women who intended to use immediate postpartum implants were seven times more likely to use highly effective birth control methods than those who intended to delay implant insertion (adjusted OR 7.1, 95% CI 1.4-35.6). Intention to breastfeed was also a predictive factor for the use of highly effective methods (adjusted OR 1.4, 95% CI 1.1-17.8; Table 5).
In the present study, the intention to undergo implantation immediately was associated with a higher utilization of contraceptive implants and highly effective contraceptive methods at 12 months. However, progestin-associated bleeding rate was higher in this group. Breastfeeding was not affected by the timing of insertion.
Only one third of the women who expressed interest in using contraceptive implants at a later date actually received them, whereas approximately 97% of women in the immediate group did so. This difference was greater than that found in a randomized trial in Uganda (99% immediate vs. 41% delayed) [12], possibly because of differences in the characteristics of participants encountered in clinical practice and those enrolled in randomized trials. The main differences included ethnicity, and the pregnancy risk status of the participants. Moreover, women who changed their minds and decided not to use implants chose either less effective contraceptive methods or no contraception. This highlights the importance of timing in contraceptive administration. A Turkish study also demonstrated a low rate of translation from intention to actual use in women with high-risk pregnancies [13]. This may be explained by the alteration of the motivation for contraceptive use over time.
At 12 months, there was higher proportion of women in the immediate group than in the delayed group who utilized contraceptive implants. However, previous randomized studies in women without high-risk pregnancies revealed no significant difference in the proportion of utilization between the groups [14,15]. We observed a much lower implant utilization in the delayed group, resulting from a low rate of initiation and participants opting for short-acting methods. None of the participants who initially decided not to use implants changed their minds later. This may be explained by differences in the motivation to use contraception, desire for pregnancy, access to birth control, and other demographic characteristics between high-risk women and their healthy counterparts [16].
In our cohort, there were only three early discontinuations in the immediate group and none in the delayed group. This is in line with the results of a United States (US) study based on Medicaid data that showed no statistically significant difference in the 12-month discontinuation of LARC by the timing of insertion [17]. The high levels of satisfaction observed in both groups may be explain this.
Unscheduled bleeding or spotting is one of the most common side effects associated with progestin-only birth control [5,18]. The proportion of women who experienced this side effect decreased over time in both groups, although it was higher in IPP implant users. However, this difference may not have clinical importance because only one participant in the immediate group underwent implant removal for this reason. This finding is consistent with those of a study conducted in the US [19]. Interestingly, the rate of dysmenorrhea was approximately double that found in our previous survey of the general population [20]. The higher proportion of women with dysmenorrhea in the immediate intervention group may be attributed to the higher rate of bleeding. Side effects other than bleeding appeared to be equivalent to those reported in low-risk postpartum women [21].
In the past, there has been a theoretical concern regarding the possible deleterious effects of early progestin contraception initiation on lactogenesis [22]. However, our findings demonstrated no negative impact of early insertion on breastfeeding, neither in terms of rate nor satisfaction. This is supported by the findings of previous studies of in women with low-risk in Uganda and Brazil [14,23].
Interestingly, according to the multivariate regression analysis, cesarean delivery was also positively associated with the use of contraceptive implants at 12 months. However, to the best of our knowledge no previous studies have reported such a relationship. We speculate that cesarean section may indicate complications from high-risk pregnancies, which might in turn have motivated these women to postpone their next pregnancy. Further research is required to confirm this association. The intention to use immediate postpartum implants is also related to the use of highly effective contraceptive methods. Similarly, Averbach et al. [12] showed a higher proportion of utilization of highly effective birth-control methods in women randomized to an IPP implant group.
Although several studies have shown that contraception affects breastfeeding, few have addressed the effect of intention to breastfeed on contraception [24,25]. We found that intention to breastfeed increased the odds of highly effective contraceptive use. This may be explained by the fact that these women were provided with information about the effects of becoming pregnant during the postpartum period, particularly with respect to lactation.
Given the paucity of data on women with high-risk pregnancies, this study fills a gap in our knowledge regarding this population. However, this study had several limitations. Unlike randomized controlled trials, retrospective studies do not allow for control of all known and unknown confounders, such as the desire for another pregnancy, or support from husband and family members. Specifically, we lacked data on why patients changed their decision to use contraceptive implants during the follow-up period. Furthermore, the relatively small sample size affected the precision of the outcomes, particularly in the analysis of the associated factors.
In conclusion, this study supports the recommendation to provide immediate postpartum contraceptive implants to women following high-risk pregnancies. Offering implants to women who intended to use this method in the immediate postpartum period appeared to improve utilization of highly effective contraceptive devices. Patients who underwent immediate insertion experienced increased spotting, prolonged bleeding, and dysmenorrhea.
Acknowledgement
We acknowledge Dr. Dylan Southard for editing the MS at the KKU Publication Clinic (Thailand).
Notes
Ethical approval
The study was approved by the Khon Kaen University Ethics Committee for Human Research (HE651105).
Table 1
Demographic data of women who received postpartum care at Srinagarind Hospital, Thailand from 2020-2021
| Intended immediate group (n=54) | Intended delayed group (n=49) | |
|---|---|---|
| Age | 27.3±6.8 | 30.2±5.9 |
| Route of delivery | ||
| Vaginal | 32 (64.0) | 28 (60.9) |
| Cesarean | 18 (36.0) | 18 (39.1) |
| Pregnancy intention | ||
| Yes | 35 (71.4) | 43 (91.5) |
| Intention to breastfeed (yes) | 35 (81.4) | 34 (77.3) |
| Level of education | ||
| Primary school | 1 (2.0) | |
| Junior high school | 12 (24.0) | 2 (4.4) |
| High school | 17 (34.0) | 13 (28.9) |
| Bachelor’s degree | 20 (40.0) | 30 (66.7) |
| Level of education (husband) | ||
| Primary school | 1 (2.1) | |
| Junior high school | 11 (22.9) | 5 (11.4) |
| High school | 20 (41.7) | 19 (43.2) |
| Bachelor’s degree | 16 (33.3) | 20 (45.5) |
| Monthly household income | ||
| No income | 3 (6.1) | 1 (2.3) |
| Less than 10,000 baht | 6 (12.2) | 2 (4.6) |
| 10,000-30,000 baht | 25 (51.0) | 24 (54.6) |
| More than 30,000 baht | 15 (30.6) | 17 (38.6) |
| Previous contraception | ||
| None | 19 (37.3) | 17 (37.0) |
| COCs | 24 (47.1) | 20 (43.5) |
| DMPA | 2 (3.9) | 3 (6.5) |
| Condom | 10 (19.6) | 5 (10.9) |
| Implant | 2 (3.92) | 2 (4.4) |
| IUD | ||
| Othera) | ||
| Maternal medical conditions | ||
| Morbid obesity | 5 (9.8) | 5 (10.9) |
| Cardiovascular disease (including congenital heart disease, valvular disease, myocardial infarction, stroke) | 1 (2.2) | |
| Cancer | 1 (2) | |
| Diabetes | 6 (11.8) | 19 (41.3) |
| Epilepsy | 1 (2.2) | |
| Human immunodeficiency virus | 2 (3.9) | 1 (2.2) |
| Systemic lupus erythematosu | 2 (3.9) | |
| Chronic renal disease/chronic liver disease | 3 (5.9) | 3 (6.5) |
| Obstetric complications | ||
| Preterm birth | 34 (66.7) | 23 (50.0) |
| Preeclampsia | 8 (15.7) | 7 (15.2) |
Table 2
Utilization of contraceptive implants at 3, 6, and 12 months in women who received postpartum care at Srinagarind Hospital, Thailand during 2020-2021, and intended to undergo immediate or delayed postpartum contraceptive implant insertion
| Intended immediate | Intended delayed | P | |
|---|---|---|---|
| Implant usage | |||
| At 3 months (n=84) | 41 (97.6) | 11 (26.2) | <0.01 |
| At 6 months (n=84) | 40 (95.2) | 11 (26.2) | <0.01 |
| At 12 months (n=83) | 38 (92.7) | 11 (26.2) | <0.01 |
| Use of highly effective contraceptive methodsa) | |||
| At 3 months (n=84) | 42 (100.0) | 27 (64.29) | <0.01 |
| At 6 months (n=84) | 40 (95.2) | 28 (66.7) | <0.01 |
| At 12 months (n=83) | 39 (95.1) | 28 (66.7) | <0.01 |
| Use of any contraceptive method | |||
| At 3 months (n=84) | 42 (100.0) | 34 (81.0) | <0.01 |
| At 6 months (n=84) | 40 (95.2) | 34 (81.0) | 0.04 |
| At 12 months (n=83) | 39 (95.1) | 35 (83.3) | 0.08 |
Table 3
Variables and side effects of implants at 1 year in women who received postpartum care at Srinagarind Hospital in Thailand from 2020-2021, and underwent immediate or delayed postpartum insertion of contraceptive implants
Table 4
Multivariate logistic regression modelling predicting utilization of contraceptive implants at 12 months postpartum in women who received postpartum care at Srinagarind Hospital in Thailand from 2020-2021
| Factor | Adjusted OR (95% CI) | P |
|---|---|---|
| Intention to use implants | ||
| Delayed | 1 | |
| Immediate | 67.64 (13.3-343.5) | <0.01 |
| Route of delivery | ||
| Vaginal | 1 | |
| Cesarean section | 6.50 (1.51-28.07) | 0.01 |
Table 5
Multivariate logistic regression modelling predicting utilization of highly effective contraception at 12 months postpartum in women who received postpartum care at Srinagarind Hospital in Thailand from 2020-2021
| Factor | Adjusted OR (95% CI) | P |
|---|---|---|
| Intention to use implants | ||
| Delayed | 1 | |
| Immediate | 7.1 (1.4-35.6) | 0.02 |
| Intention to breastfeed | ||
| No | 1 | |
| Yes | 1.4 (1.1-17.8) | 0.03 |
References
1. Blackwell S, Louis JM, Norton ME, Lappen JR, Pettker CM, Kaimal A, et al. Reproductive services for women at high risk for maternal mortality: a report of the workshop of the society for maternal-fetal medicine, the American College of Obstetricians and Gynecologists, the Fellowship in Family Planning, and the Society of Family Planning. Am J Obstet Gynecol 2020;222:B2-18.

2. Chor J, Rankin K, Harwood B, Handler A. Unintended pregnancy and postpartum contraceptive use in women with and without chronic medical disease who experienced a live birth. Contraception 2011;84:57-63.


3. Vricella LK, Gawron LM, Louis JM. Society for maternal-fetal medicine (SMFM) consult series #48: immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol 2019;220:B2-12.

4. American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus No. 8: interpregnancy care. Obstet Gynecol 2019;133:e51-72.


5. Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice bulletin No. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2017;130:e251-69.


6. Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65:1-103.

7. Jaruamornjit Y, Kaewrudee S, Sothornwit J. Differences in postpartum contraceptive choices and patterns following low- and high-risk pregnancy. Contraception 2022;107:52-7.


8. Sothornwit J, Kaewrudee S, Lumbiganon P, Pattanittum P, Averbach SH. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev 2022;10:CD011913.

9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7.


10. World Health Organization. Family planning: a global handbook for providers: evidence-based guidance developed through worldwide collaboration [Internet] Geneva: WHO; c2018 [cited 2022 Sep 11]. Available from: https://apps.who.int/iris/bitstream/handle/10665/260156/9780999203705-eng.pdf?sequence=1
.
11. Creinin MD, Vieira CS, Westhoff CL, Mansour DJA. Recommendations for standardization of bleeding data analyses in contraceptive studies. Contraception 2022;112:14-22.


12. Averbach S, Kakaire O, Kayiga H, Lester F, Sokoloff A, Byamugisha J, et al. Immediate versus delayed postpartum use of levonorgestrel contraceptive implants: a randomized controlled trial in Uganda. Am J Obstet Gynecol 2017;217:568e1-568.e7.



13. Kiykac Altinbas S, Bayoglu Tekin Y, Dilbaz B, Kilic S, Khalil SS, Kandemir O. Impact of having a high-risk pregnancy on future postpartum contraceptive method choice. Women Birth 2014;27:254-8.


14. Carmo LSMP, Braga GC, Ferriani RA, Quintana SM, Vieira CS. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol 2017;130:100-7.

15. Bryant AG, Bauer AE, Stuart GS, Levi EE, Zerden ML, Danvers A, et al. Etonogestrel-releasing contraceptive implant for postpartum adolescents: a randomized controlled trial. J Pediatr Adolesc Gynecol 2017;30:389-94.


16. Lazenby G, Francis E, Brzozowski N, Rucker L, Dempsey A. Postpartum LARC discontinuation and short interval pregnancies among women with HIV: a retrospective 9-year cohort study in South Carolina. Contraception 2019;100:279-82.


17. Rodriguez MI, Skye M, Samandari G, Darney BG. Timing of postpartum long acting, reversible contraception was not associated with 12-month removal rates in a large Medicaid sample. Contraception 2022;113:49-56.


18. Hameed W, Azmat SK, Ali M, Ishaque M, Abbas G, Munroe E, et al. Comparing effectiveness of active and passive client follow-up approaches in sustaining the continued use of long acting reversible contraceptives (LARC) in rural punjab: a multicentre, non-inferiority trial. PLoS One 2016;11:e0160683.



19. Ireland LD, Goyal V, Raker CA, Murray A, Allen RH. The effect of immediate postpartum compared to delayed postpartum and interval etonogestrel contraceptive implant insertion on removal rates for bleeding. Contraception 2014;90:253-8.


20. Sappan R, Wattanakamolchai P, Werawatakul Y, Sothornwit J. Discontinuation of contraceptive implants within 12 months of use. Thai J Obstet Gynaecol 2021;29:198-207.
21. Phemister DA, Laurent S, Harrison FN Jr. Use of Norplant contraceptive implants in the immediate postpartum period: safety and tolerance. Am J Obstet Gynecol 1995;172:175-9.


22. Kennedy KI, Short RV, Tully MR. Premature introduction of progestin-only contraceptive methods during lactation. Contraception 1997;55:347-50.


23. Averbach S, Kakaire O, McDiehl R, Dehlendorf C, Lester F, Steinauer J. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception 2019;99:87-93.



- TOOLS