How to screen the cervix and reduce the risk of spontaneous preterm birth in asymptomatic women without a prior preterm birth
Article information
Abstract
Preterm birth (PTB) is a leading cause of perinatal morbidity and mortality globally. PTB rates have increased in South Korea despite reduction in birth rates. A history of PTB is a strong predictor of subsequent PTB and screening of cervical length between 16 0/7 weeks and 24 0/7 weeks of gestation is recommended in women with a singleton pregnancy and a prior spontaneous PTB. However, the prediction and prevention of spontaneous PTBs in women without a prior PTB remain a matter of debate. The scope of this review article comprises cervical screening and prevention strategies for PTB in asymptomatic women without a prior PTB, based on recent evidence and guidelines.
Introduction
Preterm birth (PTB) occurring before 37+0 gestational weeks is the leading cause of perinatal mortality and lifelong morbidities, with almost 15 million live preterm babies born globally [1,2]. In South Korea, PTB rates have increased from 5.9% in 2011 to 9.2% in 2021 [3]. Although the survival rate of preterm infants has increased, their morbidities, including cerebral palsy, visual and hearing impairment, chronic lung problems, and poor growth, place a burden on society and families of preterm infants [4–7]. After excluding the 20–25% of PTBs caused by iatrogenic or medical indications, 75–80% of PTBs occur spontaneously following either preterm labor (PTL) or preterm prelabor rupture of membranes (PPROM) [8]. Although spontaneous PTL is considered a syndrome rather than a single condition that results from several distinct mechanisms, the major causes include activation of intrauterine inflammatory reactions from ascending infection of the genital tract and abnormal vaginal microorganisms or maternal systemic infection, cervical insufficiency, decidual senescence, uterine overdistention and malformations, stress, vascular disorder, genetic factors, and breakdown of maternal-fetal tolerance [9–11].
Cervical insufficiency is described as dilatation and shortening of the cervix before the 37th weeks of gestation, resulting in the inability of the uterine cervix to preserve a pregnancy without signs and symptoms of uterine contractions, labor, or both [12]. It can cause PPROM and pregnancy loss after the mid-trimester or PTB. Less than 1% of the obstetric population experiences cervical insufficiency [13]. A history of PTB is a strong predictor of subsequent PTB and the screening of cervical length (CL) between 16 0/7 weeks and 24 0/7 weeks of gestation is recommended in women with a singleton pregnancy and a prior spontaneous PTB [14]. However, the prediction and prevention of spontaneous PTBs in women without a prior PTB remain a matter of debate. The need for screening and prevention of PTBs in women without a prior PTB is increasing in South Korea because of the worsening low birth rate and high PTB rates [3].
The scope of this article comprises cervical screening and prevention strategies for PTB in asymptomatic women without a prior PTB, based on recent evidence and guidelines.
Screening of cervix for prediction of spontaneous PTB
Risk factors in asymptomatic women without a prior PTB
The risk factors for spontaneous PTB include adolescent pregnancy, advanced maternal age, short interpregnancy interval, multiple pregnancies, infection, underlying maternal chronic medical conditions, undernutrition, obesity, smoking, excessive drinking, recreational drug use, excess physical activity, maternal psychological health, and genetic factors [15]. Risk factors for cervical insufficiency include any factors of cervical surgical trauma, such as conization, loop electrosurgical excision procedures (LEEP), dilatation and evacuation or curettage, induced abortion, or cervical laceration [16]. Other nonsurgical risk factors include congenital Müllerian anomalies, cervical collagen and elastin vascular disorders, and in utero exposure to diethylstilbestrol.
Measurement of CL
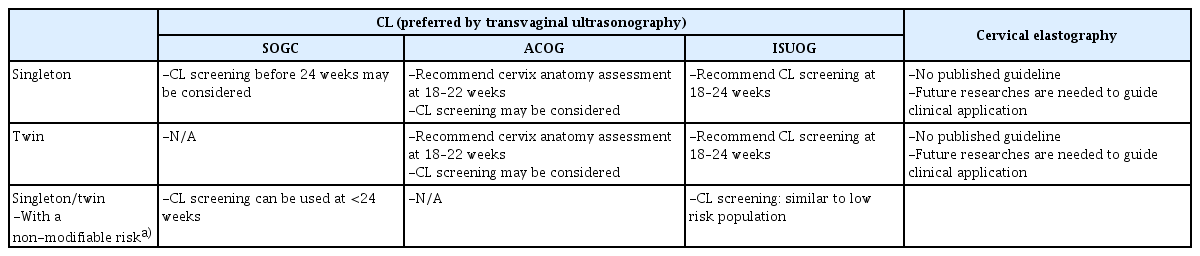
Sonographic measurement of the CL has become an essential part of screening for PTB in women with a prior PTB [16]. However, the identification of women at risk of PTB based on clinical risk factors has not been sufficient in asymptomatic women without a history of PTB [17]. Vaginal digital examination is difficult to perform during early stage cervical remodeling. In addition, because evidence of effective treatments for low-risk women with a short CL has accumulated during the last decade [18,19], several international guidelines for these women have provided different recommendations (Table 1) [20–23]. In South Korea, CL measurement has become routine, even in low-risk populations [24]. It has been recommended that CL measurement should be performed using transvaginal ultrasonography (TVS), after the maternal bladder is emptied [23,25,26]. Briefly, the cervix should occupy 50–75% of the screen, and calipers should be placed between the functional internal os and external os in the sagittal plane. The shortest length is selected from three distinct measurements in the absence of pressure need to be chosen. Owing to the thickened underdevelopment of the lower uterine segment, CL measurement in the first trimester is not recommended. To avoid misunderstanding cervical mucus as funneling, it is recommended that the amniotic membrane be observed above the internal os [23]. In addition, excessive pressure on the probe should be avoided because it can cause abnormal shortening or elongation of the cervical lips with different widths. In a meta-analysis of 57 studies, the mean CL measured using the TVS between 16 and 24 weeks was 37.96 mm (95% confidence interval 36.68–39.24), which was shorter in women from Africa and Asia, in those from low-income countries, with a lower body weight, and in those who delivered before 37 gestational weeks [21,27–29]. Transperineal ultrasound (TPUS) for CL measurement may be offered if TVS is either unacceptable or unavailable [23]. TPUS measurements can be performed by moving the abdominal probe sagittally on the perineum without introducing it into the vagina [28]. When a clear image of the cervical line is obtained, the operator completes a linear measurement from the internal orifice to the external orifice. As transabdominal ultrasound has shown significantly lower quality in the assessment of CL than TVS or TPUS, it is not recommended [14,21].
Cervical elastography
Ultrasound elastography to assess differences in tissue stiffness and elasticity has been performed in patients with liver, thyroid, or breast diseases to improve the detection of pathological problems [30,31]. In the obstetric field, CL alone using TVS has shown about 60–70% detection rate in all subsequent spontaneous PTBs [32,33]. To improve the detection rates of PTB, the E-CervixTM program (WS80A Ultrasound System; Samsung Medison, Seoul, Korea) has been developed; it uses a 6-MHz transvaginal probe to measure the stiffness of the cervical tissue and extracellular matrix. Several studies have been conducted by the Korean Research Group of Cervical Elastography [24,34–36]. Briefly, using the E-CervixTM program, the strain parameters of the internal os strain (IOS), external os strain (EOS), elasticity contrast index, IOS-to-EOS ratio, and hardness ratio can be measured after placing the region of interest caliper on the internal and external os and making the region of interest box include the entire cervix at the same mid-sagittal plane as the CL measurement with TVS [34]. Although some strain parameters measured using cervical elastography have shown significant associations with PTB [37–39], more prospective studies, including a standardized technique and beneficial management according to the findings, are needed before clinical application [40]. There are no clinical guidelines on the use of cervical elastography for cervical screening or management based on these results.
Screening of cervix and interventions for prevention of PTB in asymptomatic singleton pregnancies without a prior PTB
The American College of Obstetrics and Gynecology (ACOG), Society of Maternal-Fetal Medicine, and Society of Obstetricians and Gynecologists of Canada (SOGC) guidelines do not mandate universal CL measurement for low-risk populations but rather state that screening may be considered (Table 1) [14,20,21]. However, ACOG in 2021 stated that the cervix should be visualized at the 18 0/7–22 6/7 weeks of gestation anatomy assessment in women without a prior PTB, with either a transabdominal ultrasonography or TVS. In 2022, the International Society of Obstetrics and Gynecology (ISUOG) practice guidelines on the performance of the routine mid-trimester and the role of ultrasound in the prediction of spontaneous PTB recommended universal CL screening for PTB using TVS for asymptomatic singleton pregnancies between 18 and 24 weeks, if local economic feasibility, skills, and equipment are allowed [22,23].
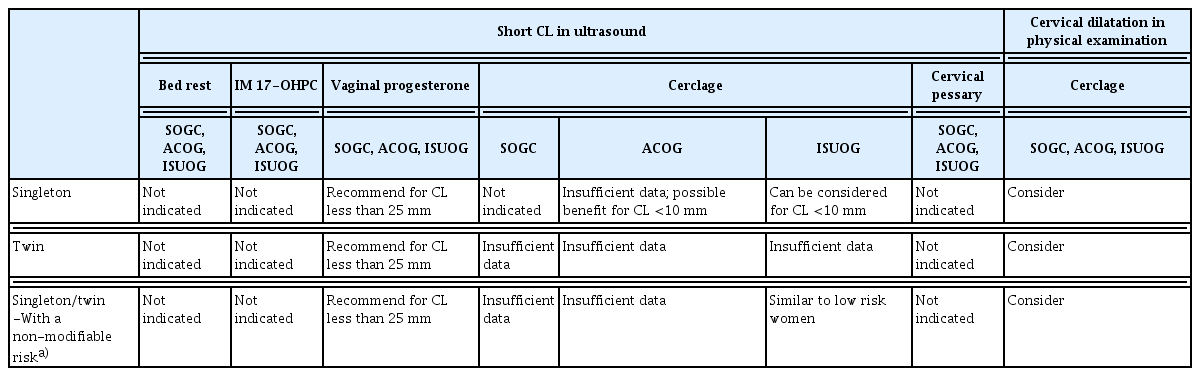
Recent meta-analyses in 2021 and 2022 reported that vaginal progesterone significantly decreased the risk of PTB <34 weeks and adverse neonatal outcomes in low-risk singleton pregnancies with short cervix (CL ≤25 mm) [19,41]. The ACOG, ISUOG, and SOGC state that vaginal progesterone administration is recommended for asymptomatic singleton pregnancies without a history of PTB but with a short cervix (less than 25 mm) [14,21,23], until 34–36 weeks of gestation (Table 2). A Cochrane meta-analysis in 2017, which included 15 randomized clinical trials (RCT) of cervical cerclage in singleton pregnancies, concluded that cervical cerclage did not influence the risk of PTB in low-risk women with a short cervix, although physical exam-based cerclage significantly reduced the risk of PTB [42]. However, a subgroup analysis of individual patient data (IPD) meta-analysis showed a significant reduction in PTB at <35 weeks in patients with CL <10 mm treated with cerclage [43]. Other studies also reported prolonged pregnancy latency and better neonatal outcomes [44,45] when the cervix was extremely shortened despite progesterone treatment in women without a prior PTB or cervical insufficiency. Although the SOGC does not recommend cerclage in asymptomatic singleton pregnancies due to insufficient evidence, the ISUOG and ACOG stated that if the CL is less than 10 mm despite progesterone treatment, cerclage can be considered. Furthermore, SOGC, ACOG, and ISUOG state that cerclage can be considered if dilatation is detected on physical examination. However, bed rest, intramuscular 17-alpha hydroxyprogesterone caproate (17-OHPC), or a cervical pessary are not recommended for singleton pregnancies in women with a short cervix and no history of spontaneous PTB [14,21,23,46]. There is no evidence that stitches at the time of cerclage can improve pregnancy outcomes compared to one stitch [47]. Although some studies have suggested that cervical pessary compared with no treatment or vaginal progesterone administration may reduce the risk of delivery before 34 weeks or 37 weeks, the certainty of evidence has been low to moderate, and further randomized controlled trials are needed [48].
Screening of cervix and interventions for prevention of PTB in asymptomatic twin pregnancies without a prior PTB
Multiple gestations are a major risk factor for PTB [49], increasing from 1.69% in 2000 to 5.4% in 2021 in South Korea [3]. Meanwhile, the risk of PTB in twin pregnancies in South Korea increased from 46.88% in 2007 to 60.93% in 2016 [50]. In asymptomatic twin pregnancies, a short cervix (CL <25 mm) before 24 weeks of gestation is the most important risk factor; other risk factors include a history of PTB, chorionicity, previous cervical operation, and conception by in vitro fertilization [51–53]. An IPD meta-analysis suggested CL <15 mm as the optimal cutoff for PTB between 28 and 32 weeks and CL <35 mm as the optimal cutoff for PTB between 32 and 36 weeks when CL is measured between 20 and 22 weeks of gestation [54]. ACOG stated that the cervix should be visualized as part of the 18 0/7–22 6/7 weeks of gestation anatomy assessment, as with singleton pregnancies, and ISUOG recommended CL measurement in asymptomatic twin pregnancies, between 18 0/7 and 24 0/7 weeks of gestation [14,23,55,56].
There is no proven strategy for preventing spontaneous PTB in asymptomatic multiple pregnancy. Cochrane meta-analyses have reported that bed rest or prophylactic oral betamimetics did not reduce the risk of PTB, but bed rest significantly increased the risk of PTB at <34 weeks of gestation [57,58]. 17-hydroxyprogesterone caproate also did not reduce the risk of PTB in asymptomatic twin pregnancies [53]. A Cochrane review in 2019 concluded that intramuscular or vaginal progesterone was not associated with a reduced risk of PTB or improved neonatal outcomes [59]. A double-blind RCT for the prevention of spontaneous PTB in twin pregnancies also reported that universal treatment with vaginal progesterone did not reduce the incidence of spontaneous PTB [60]. However, post hoc analysis demonstrated that vaginal progesterone administration may reduce the risk of spontaneous birth before 32 weeks’ gestation in women with a cervical length of <30 mm, and it may increase the risk for those with a cervical length of ≥30 mm. An IPD meta-analysis in 2022 showed that vaginal progesterone administration significantly reduced the PTB to <33 weeks in patients with twin pregnancies and a short cervix (CL ≤25 mm) during the mid-trimester [61]. The ACOG in 2021 stated that data about vaginal progesterone for the prevention of PTB is insufficient [14]. However, based on the recent meta-analysis [61], the ISUOG in 2022 suggested that prophylactic use of vaginal progesterone may be considered in asymptomatic twin pregnancy with CL ≤25 mm, although adequately powered RCTs are still needed [23].
Overall, cerclage or a pessary is not recommended in patients with asymptomatic twin pregnancies and a short CL because of conflicting results [53]. However, one RCT in 2020 and a systematic review of three retrospective case-control studies in 2019 reported that in asymptomatic twin pregnancies with a dilated cervix on physical examination, significant prolongation of pregnancy, reduction of PTB, and improvement in perinatal outcomes occurred in the cerclage placement groups compared to no cerclage groups [62,63]. The ACOG and ISUOG suggested that a combined strategy of physical-exam-indicated cerclage, antibiotics, and tocolytics may be considered in asymptomatic twin pregnancies with a dilated cervix before 24 weeks of gestation.
Screening of cervix and interventions for prevention of PTB in asymptomatic singleton or twin pregnancies without a prior PTB, but with a non-modifiable risk factor
Non-modifiable risk factors include excisional cervical treatment for cervical intraepithelial neoplasia (LEEP and conization), uterine anomalies, and multiple prior dilatation and evacuation procedures [63–66]. It is still unknown whether the increased risk of PTB is related to cervical dysplasia or cervical treatment [67]. The SOGC states that CL measurement can be used to identify an increased risk of PTB in asymptomatic women at <24 weeks with non-modifiable risk factors [68]. However, the ACOG did not specify a recommendation for CL screening in this population, although it mentioned that the risk of PTB may be increased among patients with excisions greater than 15 mm in depth and a short interval from excision to conception [69,70]. Because the ISUOG stated that CL screening in this population can be performed similarly to that in a low-risk population, CL screening at 18–24 weeks’ gestation may be reasonable. The SOGC, ACOG, and ISUOG recommended vaginal progesterone administration when the CL is less than 25 mm before 24 weeks. However, prophylactic cerclage in women with a history of cervical conization or LEEP has failed to prevent PTB, so far [71,72]. Large population-based Korean studies have demonstrated that cerclage in women with a history of cervical conization had more than twice the risk of adverse pregnancy outcomes, including PTB [73,74]. In addition, there is little evidence to support treatment in this group of women if they have a short CL on TVS. It is unclear whether cerclage is more effective than other preventive treatments such as vaginal progesterone [42,75,76]. Due to insufficient evidence to recommend specific management strategies, including cerclage or progesterone, in women with a history of cervical conization, the ISUOG suggested similar management to low-risk pregnancies without a history of cervical conization [23], but the SOGC or ACOG concluded that any recommendation about cerclage in this population is not available [14,21]. In a retrospective study from Korea, women with CL ≥20 mm at the time of cerclage who were not adherent to ACOG guideline showed significantly higher risk of PTB, neonatal composite morbidities, and severe histologic chorioamnionitis, compared with in women with CL <20 mm at the time of cerclage [62]. Because cervical dysplasia, cervical conization, or LEEP may be associated with histological alterations of the cervix, cerclage in these women needs to be decided carefully. Non-absorbable monofilament cerclage showed better prevention of PTB than braided cerclage when a short CL was observed in women with a history of conization [77,78].
Conclusion
Based on a review of recent evidence and guidelines, in asymptomatic singleton or twin pregnancies without a prior PTB, CL screening using TVS can be recommended between 18–24 weeks. In women with non-modifiable risk factors, such as cervical conization/LEEP, uterine anomalies, or multiple dilatation and curettage, CL screening can be similar to that in women without these risk factors. Although cervical elastography has shown some benefits in predicting the risk of PTB, it should not be used in clinical practice because screening and management guidelines based on more standardized research are required. When the CL is shortened to less than 25 mm, before 24 weeks of gestation, in asymptomatic singleton or twin pregnancies without a prior PTB, vaginal progesterone is recommended; however, bed rest, 17-OHPC, and cervical pessary are not indicated. If the CL is shortened to <10 mm or cervical dilatation is confirmed through physical examination despite vaginal progesterone treatment, cerclage can be considered in asymptomatic singleton pregnancies without a prior PTB, although the data are insufficient.
In asymptomatic twin pregnancies without a prior PTB, shortening of the CL (less than 10 mm) despite vaginal progesterone treatment is not an indication for cerclage due to insufficient data. However, cerclage can be considered in women if cervical dilatation is confirmed upon physical examination.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
None.
Patient consent
There is no need for patient consent in this review article.
Funding information
Not applicable.