Patient blood management to minimize transfusions during the postpartum period
Article information
Abstract
Patient blood management is an evidence-based concept that seeks to minimize blood loss by maintaining adequate hemoglobin levels and optimizing hemostasis during surgery. Since the coronavirus disease 2019 pandemic, patient blood management has gained significance due to fewer blood donations and reduced amounts of blood stored for transfusion. Recently, the prevalence of postpartum hemorrhage (PPH), as well as the frequency of PPH-associated transfusions, has steadily increased. Therefore, proper blood transfusion is required to minimize PPH-associated complications while saving the patient’s life. Several guidelines have attempted to apply this concept to minimize anemia during pregnancy and bleeding during delivery, prevent bleeding after delivery, and optimize recovery methods from anemia. This study systematically reviewed various guidelines to determine blood loss management in pregnant women.
Introduction
According to the American College of Obstetricians and Gynecologists (ACOG), postpartum hemorrhage (PPH) is defined as a cumulative blood loss of >1,000 mL or blood loss with signs or symptoms of hypovolemia within 24 hours after giving birth (including intrapartum loss) [1]. PPH is still the most common cause of maternal mortality, accounting 27% of annual postpartum deaths worldwide [2,3]. Severe PPH can result in hysterectomy, disseminated intravascular coagulation, massive transfusions, organ failure due to hypoperfusion, intensive care unit admission, and maternal death [4].
Early detection and proper intervention are essential to reduce maternal morbidity and PPH-associated mortality. The first step clinicians should take after recognizing PPH is to identify the cause of bleeding. The leading causes differ according to the timing of bleeding (Table 1) [1]. Primary PPH is described as bleeding within 24 hours after birth, and secondary PPH is described as bleeding occurring from 24 hours-12 weeks after birth [5,6]. The causes of primary PPH can be classified into “4 Ts”: tone, trauma, tissue, and thrombin (Table 2) [7]. Uterine atony, which causes approximately 70–80% of PPH cases, is the most frequent cause of primary PPH [7,8].
Early detection is vital to identify the cause of bleeding. However, detecting PPH can be challenging because postpartum women may not show typical signs or symptoms of hemorrhage, including hypotension or oliguria, unless blood loss is substantial [9]. Therefore, a strategic approach to analyzing risk factors for PPH may help improve diagnosis and clinical management. Several studies and clinical guidelines have identified various clinical risk factors for PPH, [1,10–12] and Table 3 lists well-known risk factors. However, approximately 20% of PPH cases occur in women without these risk factors; therefore, caution must be exercised [13].
Although identifying PPH risk factors and causes is critical, early and adequate PPH care is essential to lower morbidity and mortality. Various methods are available for managing PPH, including uterine massage, appropriate use of uterotonics, uterine balloon tamponade, uterine compression sutures, uterine artery embolization (UAE), and hysterectomy [14–16]. However, transfusion is an essential management method for severe PPH, regardless of the cause. Transfusion is a lifesaving intervention in patients with massive bleeding and unstable vital signs. However, excessive and unnecessary transfusions can have adverse outcomes ranging from fever and hemolytic reactions to transfusion-associated circulatory overload. Additionally, excessive transfusions can result in unnecessary socioeconomic costs. Therefore, optimizing transfusion during PPH is crucial, and implementing patient blood management (PBM) could be a solution.
Patient blood management
In PPH, the frequency of transfusion is known to be approximately 0.5–3.0% [17]. In the United States of America, the prevalence of postpartum hemorrhage increased by 26% from 2.3% to 2.6%, and the frequency of blood transfusion more than doubled; in Canada, it increased by 27% from 6.3% to 8.0%, and the frequency of transfusion increased by 40% from 17.8% to 25.5% [18–23]. In Korea, approximately 1.9% of transfusions are caused by PPH [24]. In recent years, the demand for blood transfusion for obstetric bleeding has been increasing, while the amount of blood stored for transfusion has been decreasing, owing to the coronavirus disease 2019 pandemic. The decrease in blood storage has caused a large number of delays in regular surgery worldwide [25–27]. Therefore, PBM implementation for PPH is important.
PBM is gaining traction as a solution for the potential risks of transfusion reactions and insufficient blood storage. PBM is the timely application of evidence-based medicine to maintain hemoglobin levels through optimal hemostasis and minimal blood loss and improve patient prognosis [28]. The World Health Organization (WHO) defines PBM as “the transfusion of safe blood products to treat a condition that leads to significant morbidity or mortality that cannot be prevented or managed effectively by other means, as well as the appropriate use of blood products” [29]. Many studies have shown that PBM reduces transfusion rate, intraoperative bleeding, intraoperative morbidity and mortality, and hospital stay costs [30–39]. Based on this evidence, the WHO has recommended its early implementation since 2010 [40], and the Department of Obstetrics and Gynecology of Korea University, Anam Hospital, has implemented it since 2020. We evaluated the adequacy of each transfusion for PPH based on PBM; after implementation, the rate of inappropriate transfusion for PPH decreased dramatically from 79.7% to 31.1% [41] (Fig. 1).
PBM is a practice that starts preoperatively and continues until after surgery [42]. Based on this, the three pillars of obstetric PBM can be outlined as follows: prediction and correction of prenatal anemia; prevention and reduction of hemorrhage during delivery; and finally, limited use of blood transfusion and optimizing treatment of postpartum anemia [43]. Using these three pillars, a more strategic approach should be considered, rather than choosing blood transfusion as the first-line treatment to correct low maternal hemoglobin levels.
Management of prenatal anemia
Anemia affects 40% of pregnant women globally [44]. Anemia during pregnancy is mainly due to physiological and hemodynamic changes that prevent blood loss during delivery. During the first trimester of pregnancy, plasma volume begins to increase, accompanied by hemodilution of hemoglobin [45]. Simultaneously, the number of red blood cells (RBC) increases, providing sufficient oxygen to the pregnant woman and the fetus, and preparing for any bleeding that could occur during delivery [46–48]. Consequently, the need for iron increases. As a result, iron-deficiency anemia makes up around 50% of instances of anemia during pregnancy [49]. According to the ACOG, risk factors for iron-deficiency anemia in reproductive-aged women include a low-iron diet or highly diminished iron absorption from food, malabsorption due to gastrointestinal disorders, heavy menstrual volume, and short interpregnancy intervals [50].
Therefore, the first pillar of obstetric PBM is the detection and correction of prenatal anemia. According to the WHO, anemia in pregnant women is defined as a hemoglobin level below 11 g/dL [44]. However, different cutoff values for anemia during pregnancy have been proposed based on different situations. Due to plasma volume expansion up to the second trimester of a normal pregnancy, consideration of a maximum decrease of approximately 0.5 g/dL in hemoglobin levels is recommended for diagnosis of anemia [48].
Several clinical guidelines, such as the National Institute for Health and Care Excellence (NICE), ACOG, and WHO guidelines, recommend screening for anemia for all pregnant women. Pregnant women with mild-to-moderate anemia, even those that are asymptomatic, should be evaluated using medical history, physical examination, and serum iron and ferritin levels [50–52]. According to the United Kingdom (UK) guidelines, supplementation with 40–80 mg of iron every morning is recommended for pregnant women diagnosed with anemia, and their response should be evaluated by checking hemoglobin levels every 2–3 weeks [53]. In addition, for pregnant women at high risk of iron-deficiency anemia, iron supplementation of 40–80 mg is recommended when serum ferritin is <30 mg [54]. Additionally, the Centers for Disease Control and Prevention (CDC) guidelines recommend that pregnant women diagnosed with anemia should take an iron supplement of 60–120 mg [55].
However, the prescription of iron supplements in pregnant women with normal hemoglobin and serum ferritin levels remains controversial. The WHO, CDC, and ACOG recommend a low-dose iron supplement of 30–60 mg/day for non-anemic pregnant women to prevent anemia after the third trimester of pregnancy and postpartum anemia caused by bleeding during delivery [50,52,55]. However, countries such as the UK and Switzerland do not recommend iron supplementation in non-anemic pregnant women, because it does not seem to affect perinatal outcomes [53,54].
Moreover, oral iron supplementation may not be effective because of intolerance to gastrointestinal side effects, intestinal malabsorption, or poor compliance [56]. In this case, intravenous iron is recommended [57,58]. In addition, administering an erythropoiesis-stimulating agent may be considered in patients with inadequate responses to intravenous iron [59]. Transfusion during pregnancy may be considered in cases of severe anemia with hemoglobin levels <6 mg/dL [50,51]. However, it is unclear whether transfusions are beneficial for pregnant women without other bleeding risks [59].
Management during delivery
Obstetric hemorrhage is relatively common and life-threatening. Therefore, medical staff providing obstetric care during delivery should be prepared to address the risk of a hemorrhage. The risk factors for PPH are mostly known to occur during pregnancy. Thus, pregnant women with these risk factors are advised to undergo delivery at a center where all necessary treatment can be provided on-site [43]. In addition, PPH can occur even in pregnant women without risk factors; therefore, diligent monitoring is always necessary, even at low risk [1].
Active management during the third stage of labor is recommended to prevent PPH [7]. Therefore, clinicians advise controlled umbilical cord traction, which is applied to the cord with counter-pressure on the uterus to deliver the placenta, uterine massage following placental delivery, and oxytocin administration during or immediately after delivery [60].
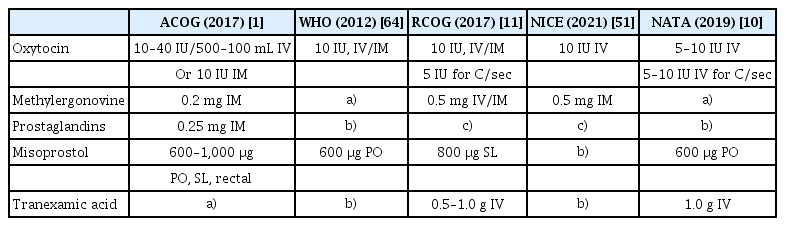
The use of uterotonics is recommended during the third stage of labor to reduce the risk of PPH [1,10,11,51,61–64]. Oxytocin (10 units, ous/intramuscular) is recommended as a first-line uterotonic. If oxytocin is not available, other injectable uterotonics (e.g., ergometrine/methylergometrine) or oral misoprostol (600 μg) can be used as an alternative. In addition, for women undergoing delivery via cesarean section, slow intravenous injection of 5 units of oxytocin is recommended. However, according to the Cochrane review, uterine massage and controlled umbilical cord traction did not prevent PPH or decrease bleeding volume [61,65].
Carbetocin is also recommended for use as a uterotonic [11,64]. It is a synthetic octapeptide analog of oxytocin, exhibiting properties similar to those of oxytocin receptor agonists, and has a significantly longer half-life than oxytocin approximately 4–10 times. This enables prolonged oxytocic effects and reduces the requirement for supplemental uterotonic agents during the postpartum period [66]. However, carbetocin is priced higher than oxytocin, misoprostol, and other uterotonics [67,68]. Therefore, its use is only recommended when it is cost-effective, considering the balance between cost and efficacy [64].
Tranexamic acid (TA) is a well-known agent that aids in reducing bleeding, with studies showing that its use in general surgery diminishes bleeding and reduces the need for RBC transfusion by one-third [69]. Furthermore, when administered within the first 3 hours of bleeding, TA has been reported to be highly effective in pregnant women with PPH [70]. However, the effect of TA requires further studies, and large-scale studies on this effect are currently underway [71,72]. Nevertheless, TA (0.5–1 g) administered intravenously with oxytocin may reduce the risk of PPH [1,11,51,64].
Uterine balloon tamponade may be used when uterine contractions cannot be sustained and bleeding cannot be controlled despite medical treatment. A Bakri balloon filled with fluid and placed within the uterus was used to compress the uterine wall and stop bleeding. Of the women who used balloon tamponades, 75% did not require further treatment or operation [6]. Balloon tamponades can assist in stabilizing bleeding until alternative therapies are available, even if severe bleeding cannot be fully controlled. Balloon tamponade is helpful in countries where other effective PPH treatments are unavailable; therefore, their use is widely recommended [1,11,51,64].
UAE is usually performed in patients after noninvasive treatment has failed, and is an effective intervention to stop bleeding following PPH [73], with a success rate of over 80% [74]. Complications such as uterine necrosis, deep vein thrombosis, and peripheral neuropathy have been reported, but the risk is low [6], and the procedure is not known to affect the next menstrual cycle or fertility [75].
Additionally, surgical bleeding during a cesarean section must be prepared for. When atonic bleeding occurs during a cesarean section, vascular ligation may be used to lower the pulse pressure of the blood entering the uterus. Bilateral uterine artery ligation (O’Leary sutures) is usually performed with a median success rate of 92% [6,76,77]. Vascular ligations are generally known not to affect uterine and reproductive functions [78]. Since iliac artery ligation requires a skilled surgeon, it has been replaced by easier-to-perform procedures such as uterine artery ligation, uterine compression sutures, and UAE [79].
Uterine compression sutures were used if no active bleeding vessels were observed. The B-Lynch suture, introduced in 1997, is the most widely used uterine compression suture method [16], after which the Hayman, Cho, Pereira, Ouahba, and Hackethal sutures were introduced [16,80]. There are no high-quality randomized trials comparing these sutures with other hemostatic methods. Its effectiveness is approximately 60–75% for intensive uterine bleeding that does not respond to medical management [16,80,81].
If all other methods fail, a hysterectomy may be considered. These are mainly recommended when hemodynamic instability and severe coagulation deficiency are observed, along with persistent bleeding despite appropriate treatment. Hysterectomy can lead to a definitive loss of fertility and potential surgical complications [6]. Therefore, it should be considered only as a last resort and sufficiently discussed with an experienced clinician [82].
Another traumatic cause of bleeding during pregnancy is birth canal laceration, with venous bleeding being the most common. However, in some cases, a tear in the uterine artery may leads to massive postpartum bleeding. To effectively manage trauma, the cause of bleeding must be identified quickly and repaired. Additioally, anesthesia assistance and a fully equipped operating room may be required to aid in repair [1,7].
Most pregnant women show typical vital signs until significant blood loss occurs due to increased plasma volume and RBC mass during pregnancy [44–47]. Therefore, vital signs during and after delivery are not a reliable measure of bleeding during labor. Transfusion preparations should be performed immediately if persistent bleeding (corresponding to a blood loss of >1,500 mL during delivery) or abnormal vital signs are observed [83,84]. This massive hemorrhage can cause wasting coagulopathy, classified as disseminated intravascular coagulation.
The optimal timing of PPH transfusion has not yet been defined. However, several protocols and clinical reports have been published on optimal transfusion timing and blood product replacement therapy in response to obstetric bleeding [83,85–90]. Massive transfusions require at least 10 packed RBC units to be administered within 24 hours, or four packed RBC units to be administered within an hour. Transfusion of packed RBCs, freshly frozen plasma, and platelets in a 1:1:1 ratio is recommended when performing massive transfusions [91]. However, this transfusion modality may not necessarily improve PPH prognosis. Further, fresh-frozen plasma administered at the beginning of PPH may be related to transfusion-associated circulatory overload and transfusion-related acute lung injury [92,93]. The clinical guidelines of the Royal College of Obstetricians and Gynecologists (RCOG) recommend administration of fresh-frozen plasma in cases where bleeding is not ameliorated even after the use of four units of RBCs [11]. Moreover, most clinical guidelines recommend that patients expected to require these blood components place an order immediately [1,11,51,64].
If compatible packaged blood cells require time to be prepared, large amounts of crystalloid or colloidal solutions may be used. Using up to 2,000 mL of warm isotonic crystalloids and an additional 1,500 mL of warm colloids is classically recommended when packed RBCs are not ready for transfusion. Currently, no randomized controlled trials have been conducted on the type of fluid used in PPH; however, the WHO recommends isotonic crystalloids rather than colloids. Nevertheless, administering sufficient fluid quickly in PPH is more important than choosing the fluid to be used. Hypothermia occurs after extensive bleeding and causes acidosis and coagulation disorders. To prevent this, it is recommended to warm the administered fluid [11,66,94].
Acidosis interferes with the hemostasis process and increases mortality rates [95]. In animal models, severe acidosis significantly inhibits the propagation phase of thrombin generation [96]. Since a complex mechanism is involved between hemostatic factors and bleeding, acidosis correction cannot recover coagulation disorders [96]. Moreover, evidence supporting the recommendation for correcting acidosis in PPH is limited due to a lack of relevant clinical studies on correction of acidosis in pregnant women. Although correcting acidosis alone cannot correct coagulopathy, it is recommended as it may aid in the process [10].
Fibrinogen is essential for achieving effective hemostasis. Fibrinogen levels are reportedly associated with PPH severity [93,97–99]; however, their measurement is time-consuming, which reduces their utility in ongoing PPH. Nevertheless, several societies, such as the RCOG and the European Society of Anesthesiology, recommend early administration when fibrinogen levels are <2 g/dL [11,99].
Recombinant factor VIIa is vital in blood coagulation and is used to treat hemophilia. However, its use as the primary treatment modality for PPH remains controversial, given its ability to lead to life-threatening thromboses [1,11,51,64].
Patients at high risk of bleeding, such as those with placenta previa and placenta accreta, can benefit from intraoperative cell salvage or autologous blood transfusions. In addition, patients who refuse to undergo blood transfusions, such as Jehovah Witnesses, can benefit from these procedures. Although there have been no large-scale prospective studies on intraoperative cell recovery in obstetric patients, it is effective and safe, and has been approved by the ACOG, RCOG, and NICE for pregnant women [1,11,51]. However, hospitals must be equipped with appropriate personnel and equipment, and high-quality filtration technology is necessary to dispel amniotic fluid contamination [100,101].
Acute normovolemic hemodilution (ANH) is similar to cell salvage therapy. ANH begins with rapid and controlled removal of the patient’s whole blood immediately before surgery. Surgery is then performed in the hemodiluted state, which results in the loss of fewer RBCs; the blood is reinfused into the patient postoperatively [102]. This allows for adequate hematocrit without additional transfusions. However, unlike cell salvage, the use of ANH in pregnant women has not been thoroughly studied, and few case reports exist of effective ANH in patients with placenta previa who underwent scheduled cesarean procedures [103,104]. Further elucidation is required to integrate ANH as a preventative approach for transfusion complications, and to reduce intraoperative blood transfusion requirements in pregnant women.
Management for the postpartum period
Bleeding occurring 24 hours after delivery is defined as secondary PPH, and appropriate management of this complication is vital [5,6]. Infection or retained products of conception (RPOC) are the most common causes of secondary PPH [1,7].
Therefore, pelvic ultrasonography may be helpful in the diagnosis of RPOC to determine the cause of secondary PPH. According to a Cochrane review published in 2002, there are no specifically recommended treatments for secondary PPH [5]. Moreover, appropriate treatment methods should be selected based on the patient’s condition and situation [1,10,11,51,64]. Although misoprostol and ergometrine may be effective, evidence supporting their use is limited [5,11]. Ultrasound-guided removal of RPOC should be carefully considered because of the risk of uterine perforation, and should be performed or supervised by an experienced clinician [11]. Bakri balloon tamponade or UAE may be employed in secondary PPH with ongoing bleeding [1,10,11,51].
Postpartum endometritis is a well-known cause of secondary PPH. According to a case series by Pather et al. [105], positive results were obtained in approximately 52% of vaginal swabs from women with secondary PPH. Group A beta-hemolytic streptococci (GAS, also known as streptococcus pyogenes), are the most common causative agents of endometritis. Extended-spectrum beta-lactamase and methicillin-resistant staphylococcus aureus infections have also increased [106]. Therefore, ampicillin alone or in combination with metronidazole is recommended to treat endometritis. However, due to the increased failure rate of ampicillin treatment, cephalosporins or clindamycin are recommended as alternatives [107]. A Cochrane review concluded that intravenous therapy with a combination regimen of clindamycin and gentamicin was clinically valuable for treating postpartum endometritis [108]. However, even with an adequate antibiotic regimen, symptoms such as abdominal pain, fever (>38°C), and tachycardia (>90 beats/min) indicate the need for administration of broad-spectrum antibiotics such as piperacillin/tazobactam or carbapenems [106].
Postpartum anemia mainly presents as iron deficiency anemia and is known to occur in about half of pregnancies after delivery [109]. The decision to check hemoglobin levels after delivery depends on levels before delivery, degree of bleeding during delivery, and peripartum symptoms [54]. According to the Swiss Society of Gynecology and Obstetrics, postpartum anemia is defined as a hemoglobin level of <12 g/dL, and <10 g/dL is defined as clinically significant anemia [54]. In addition, the network for the advancement of patient blood management, hemostasis, and thrombosis defines postpartum anemia as hemoglobin levels of <10 g/dL within 24–48 hours of delivery, <11 g/dL after 1 week, or <12 g/dL after 8 weeks [59,110]. Postpartum anemia is usually caused by prenatal iron deficiency or acute bleeding during labor, leading to decreased physical and cognitive abilities, infections, impaired breastfeeding, increased risk of postpartum depression, and decreased postpartum capacity [111–116]. Thus, it is critical to promptly recognize and address risk factors for this condition.
Oral iron supplementation is recommended for postpartum hemodynamically stable women with asymptomatic or mild-to-moderate anemia [114]. However, intravenous iron has been proven more effective, especially for women with moderate-to-severe anemia [115,116]. Packed RBC transfusion may be recommended for women with postpartum hemoglobin levels <6 g/dL, or 7–9 g/dL accompanied by symptoms of anemia. However, in the absence of active bleeding, limited transfusion is recommended. After transfusion of one packed RBC unit, post-transfusion hemoglobin level measurements and clinical evaluations were performed to determine whether additional transfusion was required. Transfusion is rarely required for hemoglobin levels >9 g/dL [50,51,54].
Conclusion
PPH is the leading cause of maternal mortality worldwide. In 20% of cases of PPH, there are no observable risk factors that may alert obstetricians and gynecologists. Blood transfusions efficiently reduce PPH-associated mortality. However, they may result in severe side effects such as circulatory overload and acute lung damage. The three pillars of obstetric and gynecologic PBM help predict and prevent bleeding due to PPH, and reduce the amount of bleeding.
First, anemia should be assessed during pregnancy, and iron supplementation should be recommended if necessary. Furthermore, risk factors for PPH should be analyzed, high-risk patients should be identified, and delivery in advanced medical centers should be encouraged.
Second, uterotonics should be administered to all pregnant women during delivery. The guidelines for uterotonic use are listed in Table 4. Appropriate methods, such as balloon tamponade, uterine compression sutures, and UAE, should be used to minimize blood loss if PPH occurs. Additionally, appropriate transfusions should be performed when necessary.
Third, it is recommended to check for postpartum anemia if prepartum anemia, massive bleeding during delivery, or symptoms of anemia during the peripartum period are observed. Depending on its severity, oral iron supplements, intravenous iron, or packed RBC transfusions are recommended.
Ordinary blood transfusions can be reduced through appropriate measures, and bleeding during and after delivery can be minimized.
Notes
Conflict of interest
All authors have no potential conflicts of interest.
Ethical approval
None.
Patient consent
There is no need for patient consent in this review article.
Funding information
None.