The effect of antioxidant supplementation on dysmenorrhea and endometriosis-associated painful symptoms: a systematic review and meta-analysis of randomized clinical trials
Article information
Abstract
This study aimed to review randomized controlled trials (RCTs) investigating the effects of dietary antioxidant supplements on the severity of endometriosis-related pain symptoms. The PubMed/Medline, Scopus, and Web of Science databases were searched until April 2022. Additionally, we manually searched the reference lists. Endpoints were summarized as standardized mean difference (SMD) with 95% confidence intervals (CIs) in a random-effects model. The I2 statistic was used to assess heterogeneity. Ten RCTs were included in this meta-analysis. Overall, 10 studies were related to dysmenorrhea, four to dyspareunia, and four to pelvic pain. Antioxidants significantly reduced dysmenorrhea (SMD, −0.48; 95% CI, −0.82 to −0.13; I2=75.14%). In a subgroup analysis, a significant reduction of dysmenorrhea was observed only in a subset of trials that administered vitamin D (SMD, −0.59; 95% CI, −1.13 to −0.06; I2=69.59%) and melatonin (SMD, −1.40; 95% CI, −2.47 to −0.32; I2=79.15%). Meta-analysis results also suggested that antioxidant supplementation significantly improved pelvic pain (SMD, −1.51; 95% CI, −2.74 to −0.29; I2=93.96%), although they seem not to have a significant beneficial impact on the severity of dyspareunia. Dietary antioxidant supplementation seems to beneficially impact the severity of endometriosis-related dysmenorrhea (with an emphasis on vitamin D and melatonin) and pelvic pain. However, due to the relatively small sample size and high heterogeneity, the findings should be interpreted cautiously, and the importance of further well-designed clinical studies cannot be overstated.
Introduction
Endometriosis is a chronic inflammatory gynecological condition characterized by the presence and growth of tissues similar to the endometrium outside the uterine cavity. It affects at least 10% of women of reproductive age [1]. Furthermore, it frequently causes pelvic pain, dysmenorrhea, dyspareunia, and infertility, impairing patients’ quality of life [2]. Endometriosis is a multifaceted, symptomatic, pathobiological, multisystem, and heterogeneous disease. Based on the phenotype, it can be categorized into superficial peritoneal lesions, ovarian endometriomas, and deep-infiltrating endometriosis [3,4]. When addressing general endometriosis management, three therapeutic modalities are commonly available: I) medicinal treatment (e.g., painkillers, nonsteroidal anti-inflammatory drugs, combined oral contraceptives, progestins, and gonadotropin-releasing hormone analogs); II) surgery (conservative or definitive); and III) assisted reproductive technologies (such as in vitro fertilization and intra-cytoplasmic sperm injection). It is crucial to emphasize the timely administration of pain management, as therapeutic inertia portends the development of central sensitization (autonomous) [5–7]. Interestingly, reduction in oxidative stress is also a crucial alternative for endometriosis management. Inflammation leads to an increased production of reactive oxygen species (ROS), which play a fundamental role in the proliferation of endometriotic cells as well as in the development, persistence, and progression of the disease [8]. Dietary micro- and macro-nutrients and dietary factors are pivotal in controlling chronic diseases [9,10]. Dietary antioxidants exert beneficial neutralizing effects against free radicals and ROS produced by endometriotic cells. They demonstrate anti-inflammatory properties [11,12] and pro-apoptotic and anti-angiogenic actions and generally have a favorable safety profile. Therefore, one could speculate that they may effectively reduce pain-inducing factors and improve endometriosis-associated clinical symptoms [13–15].
A growing body of literature has explored the impact of different dietary antioxidants on endometriosis-related pain [15]. Antioxidant vitamins successfully reduce the intensity of dysmenorrhea, ameliorate dyspareunia and pelvic pain, and improve the quality of life in patients with endometriosis. Consequently, therapy involving antioxidant vitamins may be considered an alternative treatment approach, independently or in conjunction with other methods, to alleviate endometriosis-related pain [16]. Zheng et al. [16] showed that supplementation with vitamin E improved endometriosis-related pelvic pain, whereas supplementation with vitamin D did not. Some systematic reviews also reported no significant effects of vitamin D on dysmenorrhea or non-cyclic pelvic pain [17]. Nevertheless, no systematic reviews or meta-analyses have comprehensively summarized the effects of dietary antioxidants on endometriosis and dysmenorrhea. To bridge this gap, we conducted a comprehensive systematic review and meta-analysis of randomized controlled trials (RCTs). We aimed to investigate the duration response and the impact of administering various dietary antioxidant supplements, including vitamins D, C, E, and A, melatonin, curcumin, omega-3 fatty acids, resveratrol, zinc, copper, chromium, and selenium, separately or in different combinations, on the severity of endometriosis-associated painful symptoms. The symptoms evaluated include dysmenorrhea, dyspareunia, and chronic pelvic pain in women of reproductive age.
Methods
This systematic review and meta-analysis of RCTs was performed according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses statement and the current recommendations of the Cochrane Collaboration.
1. Search strategy and study selection
We searched electronic research databases, including Scopus, Web of Science (Science and Social Science Citation Index), and PubMed/Medline, from their inception until April 2022. In addition, we manually searched the reference lists and citations of the eight identified articles using Google Scholar. We also contacted authors who had published in this area to ensure we did not miss any relevant publications. Search terms were set by the authors and adapted for use in other databases. There were no restrictions on the language or publication dates. Document with incomplete data or the author could not be reached was discarded. The search strategy is described in detail in Supplementary Table 1. Two reviewers (S.B. and A.G.) independently screened all identified records for potentially eligible studies after reading all titles and abstracts. The final inclusion criteria were determined after reading the full texts strictly. Disagreements were resolved through discussion with a third reviewer (A.A.).
2. Eligibility criteria
The eligibility criteria of the studies were formulated according to the participants, interventions, comparisons, outcomes, and study design criteria. I) Participants: women with clinically and/or histologically confirmed endometriosis; II) intervention: supplementation with antioxidants (vitamins D, C, E, and A, melatonin, curcumin, omega-3 fatty acids, resveratrol, zinc, copper, chromium, and selenium, separately or in different combinations); III) comparators: antioxidant versus no treatment, antioxidant versus placebo; IV) outcomes: severity of dysmenorrhea, dyspareunia and/or chronic pelvic pain assessed by any pain assessment scale/tool; and V) study design: a clinical randomized controlled study. Studies meeting any of the following criteria were excluded: I) pain caused by reasons other than endometriosis; II) non-randomized clinical trials (e.g., conference abstracts, repeated publications, animal experiments, case reports, and reviews); and III) studies without available data for analysis.
3. Data extraction and assessment of the quality of included studies
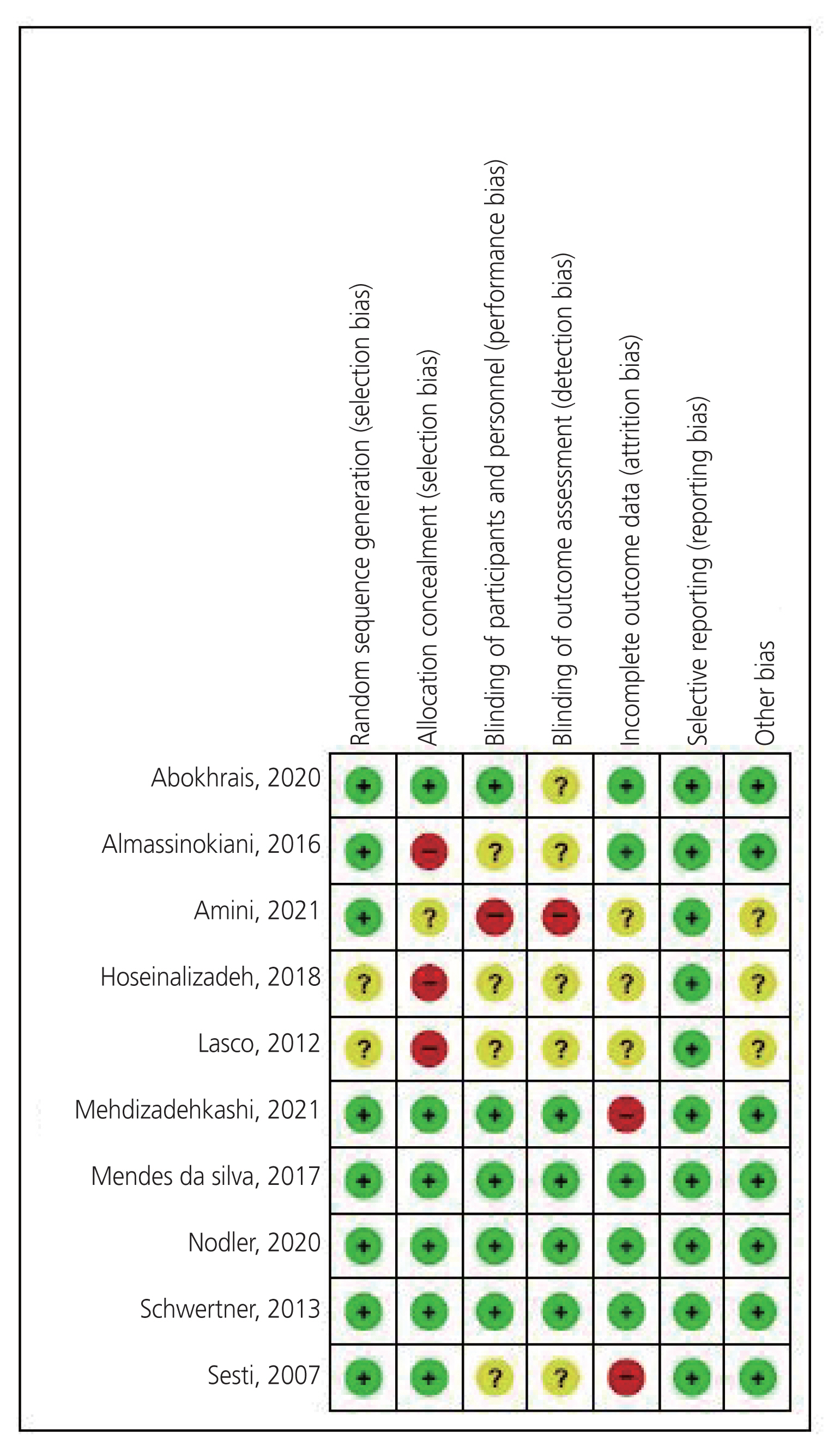
Two reviewers (H.S. and M.S.A.) independently extracted the following data from the included trials: participant characteristics (e.g., age, diagnostic method, body mass index, and parity), study characteristics (e.g., first author name, publication year, region, study design, sample size, intervention type, and intervention characteristics), and study outcomes. The corresponding authors were contacted for additional information. Two reviewers (H.S. and M.S.A.) assessed the methodological quality of the included trials according to the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (Cochrane, London, England). Disagreements were resolved through discussion with a third reviewer (K.K.). Each trial was evaluated for seven items: random sequence generation, concealed allocation, blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases; each item was rated as “high risk”, “low risk”, or “unclear”.
4. Statistical analysis
Stata version 16 (Stata Corporation, College Station, TX, USA) was used for the statistical analyses. All continuous variables were pooled by standard mean differences (SMDs) with 95% confidence intervals (CIs). Heterogeneity was evaluated using the Higgins’ I2 statistic. I2 statistics of 0–25%, 25–50%, 50–75%, and >75% were suggestive of very low, low, moderate, and high heterogeneity, respectively. We used a random-effects model to calculate individual study SMD and corresponding 95% CIs. In addition, a chi-square test for heterogeneity was performed, and the P-values were presented. Exploration of the causes of heterogeneity was planned using variations in the antioxidant type and duration of the intervention. We performed sensitivity analyses to evaluate the robustness of pooled estimations after exclusion of every single trial through the “Leave-one-out method”. Additionally, we assessed the risk of publication bias across studies using counter-funnel plots of the outcomes. We used the GRADEpro GDT software (Evidence Prime, Hamilton, ON, USA) to formally assess the quality of evidence for selected outcomes. We evaluated the outcomes considering the following five criteria: risk of bias, inconsistency, indirectness, imprecision, and publication bias, and the level of evidence was graded as very low, low, moderate, or high. The certainty of the evidence evaluation is presented in Supplementary Table 2.
Results
1. Study selection
Initially, 134 records were identified through a database search, and eight additional records were identified through other sources. All studies were then imported into EndNote X9 software (Thomson Reuters, Philadelphia, PA, USA), 25 duplicates were removed, and 91 studies were removed after the title and abstract screening process; thus, 18 studies were screened for eligibility in more detail. Finally, after detailed title, abstract, and full-text evaluations, 10 [13,18–26] RCTs were included in this systematic review and meta-analysis. A flow diagram of the complete literature search and selection of studies is shown in Fig. 1.
2. Study characteristics
The ethnicity of the study population varied among the studies: Iran (n=4), Brazil (n=2), Italy (n=2), Britain (n=1), and the United States (n=1). All the included trials, except one, were published in English between 2007 and 2021. A total of 541 women aged 20–40 years were examined, with individual study sample sizes ranging from 30 to 145 participants. Endometriosis was confirmed by histopathology in all trials. Seven trials reported the endometriosis stage in the patients. The characteristics of the included trials are summarized in Table 1. A summary of the risk of bias demonstrated that the methodological quality of these trials was relatively desirable (Fig. 2). All participants in each trial were randomly allocated to groups using an adequate allocation procedure. The randomization was unclear in both trials. Six trials stated that the allocation was concealed, one trial stated that it was unclear, and three trials were at high risk. Half of the trials used patient masking, and the blinding of participants and personnel was unclear in four trials. Thus, only one trial had a high risk of performance bias. Four trials reported appropriate blinding of the outcome assessment, whereas blinding was unclear in five trials. Only one trial had a high risk of detection bias. Half the trials provided an intention-to-treat analysis. Three trials were conducted for each protocol. Two trials had a high risk of attrition bias. All the trials were judged to have a low risk of reporting bias. Finally, three trials were judged as unclear for other biases. All trials recorded sufficient data on dysmenorrhea; however, five of these trials provided insufficient data on pelvic pain, and four studies did not provide sufficient information on dyspareunia.
3. The effect of antioxidants on dysmenorrhea
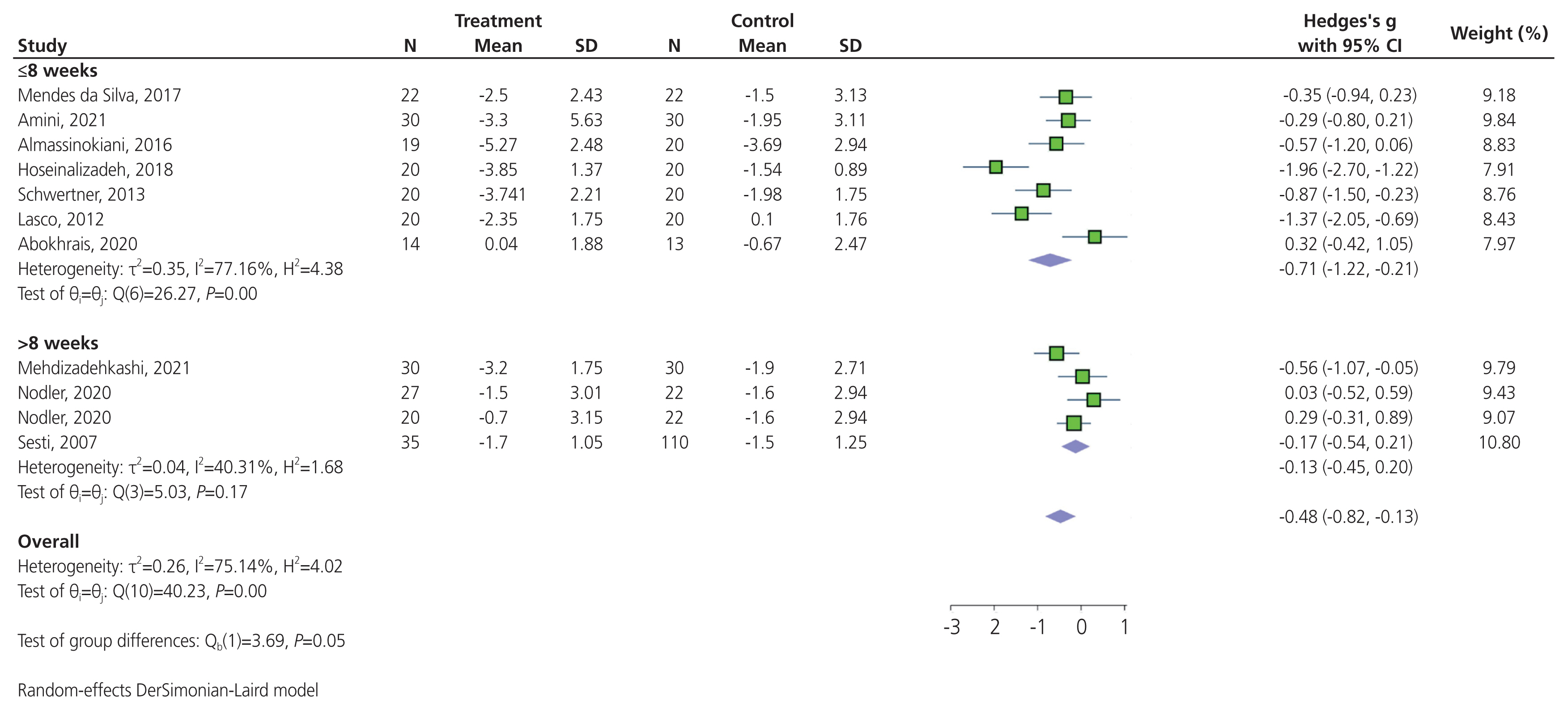
Pooling results from 10 trials that compared the effect of antioxidants versus placebo in terms of reducing dysmenorrhea suggested that antioxidants significantly reduced dysmenorrhea (SMD, −0.48; 95% CI, −0.82 to −0.13; P<0.001) (Fig. 3). High heterogeneity was detected between trials (P<0.001; I2=75.14%); however, there was no indication of publication bias affecting these findings (Egger’s regression intercept, −4.77; 95% CI, −12.17 to 2.62; P=0.178). In a subgroup analysis, a significant difference was observed in the subset of trials that compared the effect of various antioxidants on dysmenorrhea: vitamin D, omega-3 fatty acids, resveratrol, melatonin, and a combination of antioxidants (Chi2, 11.52; degree of differentiation, 4; P=0.02). There was a significant reduction in dysmenorrhea in the subset of trials that administered vitamin D (SMD, −0.59; 95% CI, −1.13 to −0.06; P=0.02; I2=69.59%), and those that administered melatonin (SMD, −1.40; 95% CI, −2.47 to −0.32; P=0.03; I2=79.15%; Fig. 3). No significant reduction in the severity of dysmenorrhea was observed in the subset of trials that administered omega-3 fatty acids (SMD, 0.30; 95% CI, −0.16 to 0.76; P=0.96; I2=0%), resveratrol (SMD, −0.35; 95% CI, −0.94 to 0.23), or combination of antioxidants (SMD, −0.21; 95% CI, −0.51 to 0.09; P=0.69; I2=0%; Fig. 3). A significant reduction in dysmenorrhea was observed in the subset of trials that administered antioxidant supplementation for ≤8 weeks (SMD, −0.71; 95% CI, −1.22 to −0.21; P<0.001; I2=77.16%). Conversely, no significant reduction was observed in the subset of trials with antioxidant supplementation longer than 8 weeks (SMD, −0.13; 95% CI, −0.45 to 0.20; P=0.17; I2= 40.31%; Fig. 4). The sensitivity analysis indicated that the exclusion of any individual trial did not significantly alter the overall results of the meta-analysis, revealing the high stability of the results (Supplementary Fig. 1). A funnel plot depicts a triangular region centered on the pooled SMD, in which 95% CI of study findings should fall if there is no publication bias and no heterogeneity in the underlying true effects (Supplementary Fig. 2).
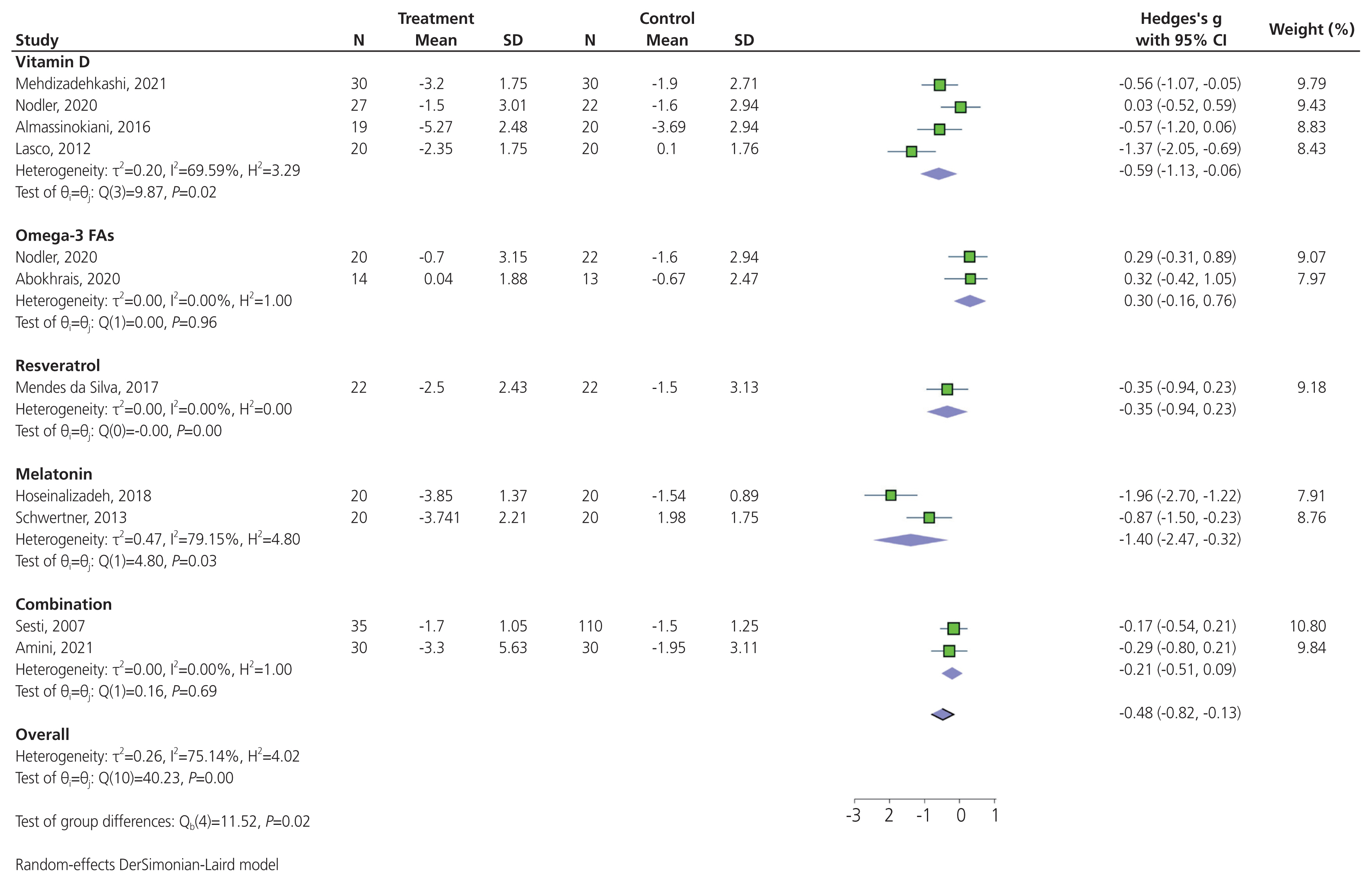
Forest plot of the effect of antioxidants on dysmenorrhea pain. N, number; SD, standard deviation; CI, confidence interval; FA, fatty acid.
4. The effect of antioxidants on dyspareunia
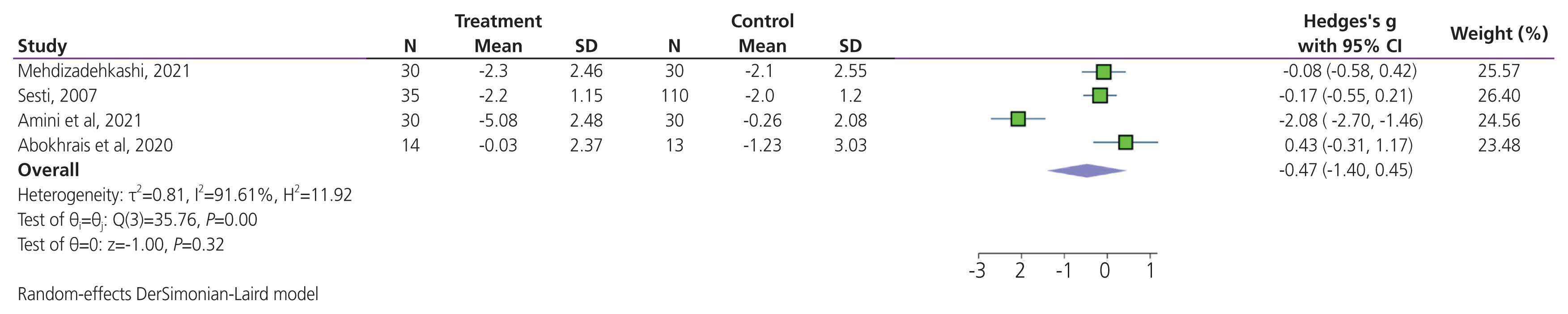
Total effectiveness was reported in four trials. Meta-analysis results suggested that there was no significant difference in terms of dyspareunia levels when comparing antioxidant supplementation and placebo (SMD, −0.47; 95% CI, −1.40 to 0.45; P=0.32; Fig. 5). High heterogeneity was detected between trials (I2= 91.61%; P<0.001). The sensitivity analysis indicated that excluding any individual trial did not significantly alter the overall results of the meta-analysis, revealing the high stability of the results (Supplementary Fig. 3).
5. The effect of antioxidants on pelvic pain
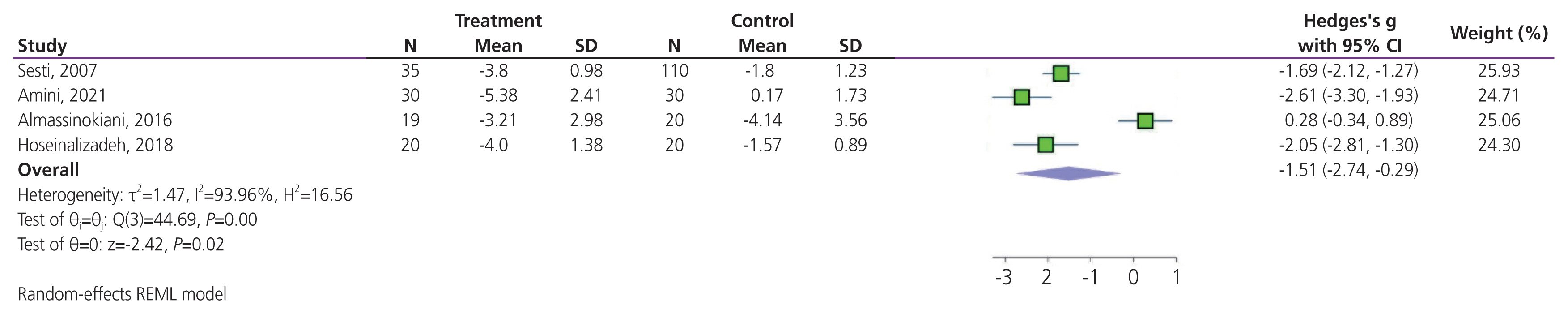
Pelvic pain was recorded in four trials. Meta-analysis results suggested that antioxidant supplementation significantly improved endometriosis-associated pelvic pain (SMD, −1.51; 95% CI, −2.74 to −0.29; P=0.01; Fig. 6). High heterogeneity was detected between trials (I2=93.96%; P<0.001). Sensitivity analysis showed that the pooled SMD varied considerably with the omission of three trials; in particular, the exclusion of the study by Sesti et al. [26], Amini et al. [13], and Hoseinalizadeh and Cahichian. [20] which accounted for approximately 25.93%, 24.71%, and 24.30% of all weights in the meta-analysis, resulted in a pooled SMD (95% CI) of −1.45 (−3.20 to 0.29), −1.15 (−2.56 to 0.26), and −1.34 (−3.00 to 0.32), respectively (Supplementary Fig. 4).
6. Quality of evidence
We were moderately confident in the outcomes of dysmenorrhea because of the uncertainty regarding the risk of bias. We had very little confidence in the outcomes of dyspareunia and pelvic pain because of some uncertainty regarding the risk of bias, inconsistency (high heterogeneity), and imprecision (non-significant results) (Supplementary Table 2).
Conclusion
Numerous studies have assessed the effectiveness of dietary and supplemental antioxidants for managing different types of pain in women with endometriosis. Previous systematic reviews and meta-analyses have reported inconsistent results. In line with our findings, systematic reviews support the positive effects of antioxidants in improving endometriosis-associated pain [16]. However, systematic reviews and meta-analyses have indicated that certain antioxidant vitamins, such as vitamin D, may not efficiently alleviate pain in patients [17]. Dysmenorrhea, dyspareunia, and chronic pelvic pain stand out as the most prevalent issues in women of reproductive age [27,28]. Our findings suggest that dietary and supplemental antioxidant intake is associated with a notable reduction in dysmenorrhea and chronic pelvic pain. However, our meta-analysis did not reveal a significant effect of antioxidant intake on dyspareunia in women with endometriosis.
Dietary antioxidants such as vitamin D, omega-3 fatty acids, and melatonin can have an interactive impact on decreasing cellular damage induced by oxidative stress and ROS [29–31]. Dietary antioxidants can neutralize the ROS and oxidative damage associated with pain in endometriosis. The involvement of oxidative stress and related markers in the initiation and progression of endometriotic complications has been suggested in experimental studies [32]. Cultivated endometrial stromal cells were treated with antioxidants and oxidative stress factors. The application of antioxidant agents resulted in a dose-dependent suppression of cell growth. In contrast, control cells treated with oxidative stress factors exhibited increased endometrial stromal growth [33]. The most accepted mechanism for this effect is an antioxidant system that removes free radicals and functions through superoxide dismutases that remove the superoxide anion and glutathione peroxidase, which then removes hydrogen peroxide. In women with endometriosis, there is a diminished function of the antioxidant system activity [34].
It has been shown that the levels of most oxidative stress parameters are markedly enhanced in women with endometriosis compared with controls, and this is one of the main factors contributing to pain in these patients [35,36]. Several recent studies have shown that oxidative stress is positively associated with the migration and proliferation of endometrial cells in the peritoneal cavity, thereby increasing the probability of endometriosis and infertility [32,37]. The correlation between ROS generation and endometriosis is verified and widely investigated [38]. Moreover, it has been demonstrated that ROS and their reactive oxygen precursors are essential in the progression of pain in several etiologies [39]. Therefore, control of ROS leads to pain relief in patients with endometriosis, and it has been widely reported that dietary antioxidant supplements can effectively decrease ROS levels [40,41]. It was also observed that the increased ROS and enhanced proliferative potential in endometriotic cells were related to the full stimulation and elevated levels of phosphorylated endoplasmic reticulum kinase (ERK), as previously detected in tumor cells [42]. In addition, enhanced ERK phosphorylation has recently been reported in stromal cells of women with endometriosis [43]. Furthermore, there are links between ROS generation, ERK activation, and endometriotic cell proliferation [44]. It was also demonstrated that ERK activation is in response to different pro-inflammatory factors such as tumor necrosis factor-α and interleukin-1β [45,46]. These two pro-inflammatory markers are enhanced in the endometrium of women with endometriosis [47,48]. Pro-inflammatory markers have a pivotal role in the progression and development of pain in patients with endometriosis [49,50]. In addition, dietary antioxidant supplements have been shown to regulate pro-inflammatory cytokines efficiently [51].
Recent studies have also shown increased concentrations of other oxidative stress parameters in women with primary dysmenorrhea. Malondialdehyde (MDA) is an index of lipid peroxides [52]. MDA concentrations were higher in women with endometriosis than in healthy controls [53]. Lipid peroxide levels were higher in peritoneal fluid samples from women with endometriosis than in healthy controls [34]. Therefore, a decrease in MDA levels may help control cell proliferation in endometriosis, and it has been demonstrated that dietary antioxidants such as vitamin D and omega-3 fatty acids can decrease MDA levels [29,54].
Recent investigations have reported the elevated level of iron in different parts of the peritoneal cavity of women with endometriosis, including endometriotic lesions, macrophages, and peritoneal fluid, which basically proposes dysfunction of iron homeostasis in the peritoneal environment among this group of patients [55,56]. In women with endometriosis, elevated iron levels may result from the lysis of pelvic erythrocytes [57]. Retrograde menstruation leads to severe hemolysis of erythrocytes accompanied by a disruptive or overwhelmed peritoneal elimination system, which enhances iron levels in the peritoneal environment, leading to the progression and growth of endometrial cells [58,59]. Iron overload might have several cytotoxic effects because it reduces the equilibrium between free radical generation and the antioxidant system, which can cause oxidative stress [60]. Our subgroup analysis indicated that vitamin D and melatonin were more effective in decreasing dysmenorrhea in patients with endometriosis. It has been demonstrated that vitamin D [61] and melatonin can efficiently decrease iron toxicity complications [62,63].
The effectiveness of vitamin D in reducing dysmenorrhea in patients with endometriosis is thought to be mechanistically linked to its anti-inflammatory and immunomodulatory properties [64]. Endometriosis is characterized by endometrial-like tissue outside the uterus, causing inflammation and pain during menstruation [65,66]. Vitamin D receptors exist in various cells, including those involved in immune responses and inflammation [67]. By binding to these receptors, vitamin D may regulate the production of inflammatory mediators such as cytokines and modulate immune system activity [68]. This regulatory effect is believed to mitigate the heightened inflammatory response associated with endometriosis and subsequently reduce the severity of dysmenorrhea [69]. Although the precise mechanisms are still under investigation, emerging research suggests that maintaining optimal vitamin D levels may play a role in managing the pain and inflammation associated with endometriosis-related dysmenorrhea. On the other hand, melatonin, primarily recognized as a regulator of the sleep-wake cycle, also exhibits potent antioxidant properties [70]. Melatonin supplementation may be beneficial in the context of endometriosis-related dysmenorrhea, where oxidative stress is pivotal to exacerbating pain and inflammation [25]. Endometriosis is associated with increased ROS production, contributing to tissue damage and increased pain sensitivity [8,71]. Melatonin functions as a free radical scavenger and mitigates oxidative stress by neutralizing harmful molecules [72]. Therefore, the antioxidant effect of melatonin holds promise in reducing the severity of dysmenorrhea in patients with endometriosis [73]. By alleviating the oxidative burden, melatonin may contribute to the modulation of inflammatory responses and a subsequent decrease in pain perception, offering a supplementary avenue for managing the discomfort associated with endometriosis-related menstrual pain [74].
Additionally, in the same context, vitamins C and E, similar to vitamin D and melatonin, are recognized for their role in regulating iron levels, preventing excess, or addressing deficiency. This regulation aids in reducing oxidative stress because these vitamins function as antioxidants. However, perhaps due to the limited number of included studies that assessed the effectiveness of vitamins C and E, coupled with heterogeneous findings, we could not identify significant results for these vitamins. Therefore, further large-scale studies are required.
1. Strengths and limitations of the current review
This systematic review and meta-analysis has several limitations. The small number of included trials makes it difficult to draw a definitive conclusion regarding the effect of antioxidant supplements on dyspareunia in patients with endometriosis; therefore, these results should be interpreted cautiously. In addition, there are not enough trials in the literature to cover all dietary antioxidant supplements that may effectively reduce endometriosis-associated pain symptoms. Another limiting factor in the findings of this meta-analysis was the heterogeneity of the study populations and the types of antioxidants included in the trials. Some researchers failed to provide sufficient information on the selection criteria and data regarding where the trials were performed.
Dietary antioxidant supplementation seemed to have a beneficial effect on the severity of endometriosis-related dysmenorrhea (with an emphasis on vitamin D and melatonin) and pelvic pain. In contrast, no significant reduction in dyspareunia was observed. However, the obtained findings should be interpreted cautiously owing to the relatively small sample size and high heterogeneity between studies, and the importance of further well-designed clinical studies cannot be overstated.
Supplementary Information
Notes
Conflict of interest
All authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Patient consent
Not applicable.
Funding information
None.