Vulval premalignant lesions: a review article
Article information
Abstract
Vulvar intraepithelial neoplasia (VIN) is a noninvasive squamous lesion that is a precursor of vulvar squamous cell cancer. Currently, no screening tests are available for detecting VIN, and a biopsy is performed to confirm the clinical diagnosis. Despite sharing many risk factors with cervical intraepithelial neoplasia, the diagnosis of VIN is poses challenges, contributing to its increasing prevalence. This study aimed to analyze the underlying risk factors that contribute to the development of VIN, identify specific populations at risk, and define appropriate treatment approaches. Differentiated VIN (dVIN) and usual VIN (uVIN) are the classifications of VIN. While dVIN is associated with other vulvar inflammatory disorders, such as lichen sclerosis, the more prevalent uVIN is associated with an underlying human papillomavirus infection. Patients with differentiated VIN have an increased risk of developing invasive malignancies. Few effective surveillance or management techniques exist for vulvar intraepithelial neoplasia, a preinvasive neoplasm of the vulva. For suspicious lesions, a thorough examination and focused biopsy are necessary. Depending on the specific needs of each patient, a combination of surgical and medical approaches can be used.
Introduction
Squamous vulvar intraepithelial neoplasia (VIN) is a premalignant skin disorder that often causes severe and long-lasting pruritus, pain, and psychosexual dysfunction. It exhibits a spectrum of clinical and histopathological manifestations and is categorized into two subtypes, usual type VIN, caused by a persistent infection with high-risk human papillomavirus (HPV), and differentiated type VIN, associated with lichen sclerosus (LS).
History of evolution of classification system of VIN
Since Bowen’s 1912 description of squamous intraepithelial lesions, several names have been used. Kaufman (1965) classified premalignant lesions into the following three groups: carcinoma simplex, Queyrat’s erythroplasia, and bowenoid carcinoma in situ [1]. In 1976, the International Society for the Study of Vulvar Disease (ISSVD) replaced all terminologies with vulvar atypia and carcinoma in situ [2] (Table 1). Subsequently, a decade later, these terminologies were replaced with vulvar intraepithelial neoplasia [3].
Similar to cervical intraepithelial neoplasia, VIN was classified into the following three subtypes: VIN 1 (mild dysplasia), VIN 2 (moderate dysplasia), and VIN 3 (severe dysplasia) [3]. This grading scheme implies that the VIN lesions are part of a biological continuum. However, clinicopathological evidence does not support the existence of such a continuum. Therefore, the ISSVD abolished the grading scheme in 2004 and replaced it with a two-tiered classification for squamous VIN, including usual- and differentiated-type VIN. These two types differ based on their etiology, morphology, biology, clinical characteristics, and malignant potential [4,5]. Nonetheless, the three subcategories of the World Health Organization (WHO) classification, VIN 1, 2, and 3 are still commonly used [6].
Histologically, usual vulvar intraepithelial neoplasia (uVIN) encompasses warty, basaloid, and mixed (warty/basaloid) VINs. Persistent infection with high-risk or oncogenic HPV (mostly HPV types 16, 18, and 33) typically cause of uVIN [7]. This subtype predominantly affects younger women and exhibits a multifocal pattern. Although less common, approximately 2–5% of all VIN lesions are of the differentiated type, differentiated vulvar intraepithelial neoplasia (dVIN) has the highest potential for malignancy [1,8]. It is linked to LS but is unrelated to HPV and typically affects older women [9]. Most often, the dVIN is unicentric and has a strong correlation with co-existing invasive vulvar squamous cell carcinoma [10,11].
In addition to changing the VIN classification, the ISSVD modified the grading system. While it was established that VIN 1 occurs only in the condylomata acuminata, additional studies showed an overlap in the diagnosis of VIN 2 and VIN 3. Furthermore, it has been shown that the pathologic diagnosis of VIN 1, 2, and 3 lacked reproducibility, although the pathologic diagnostic of VIN 2 and 3 combined is more reproducible [12,13]. Currently, only histologically “high-grade” squamous lesions (VIN 2 and VIN 3) are classified as VINs, and VIN 1 is no longer recognized.
The 2013 lower anogenital squamous terminology (LAST) uses a two-tier terminology system, classifying lesions as “low-grade squamous intraepithelial lesions (LSIL)” and “high-grade squamous intraepithelial lesions (HSIL)” of the vulva and other genital organs. This system unifies the nomenclature of squamous lesions associated with the HPV throughout the lower anogenital tract [12]. However, the main limitations of the LAST classification are the inclusion of vulvar LSIL, which has the potential for overdiagnosis and overtreatment of benign and usually self-limiting lesions, and the absence of reference to dVIN, despite its malignant potential.
The term “carcinoma in situ” of the vulva is still used in the 2018 International classification of diseases for mortality and morbidity statistics, 11th revision (international classification of diseases-11) system [13] for both squamous and non-squamous preinvasive lesions (Paget’s disease), where the possibility of impending cancer may prompt needless radical excisions of every intraepithelial neoplastic lesion. The terms HSIL and LSIL are included in the latest 2015 ISSVD terminology (Table 2) [14]. However, the word “neoplasia” has been replaced with the word “lesion”, and LSIL has been described as the manifestation of a flatter condyloma or HPV effect, while the third group remains “vulvar intraepithelial neoplasia differentiated”, as stated in the previous ISSVD terminology

The 2015 International Society for the study of vulvovaginal disease terminology of vulvar squamous intraepithelial lesions
In 2014, the WHO classified squamous intraepithelial lesions using three categories, including LSIL (low grade), HSIL (high grade), and “VIN-differentiated type” [15]. In contrast, the 2020 WHO tumor classification [16] divides vulvar lesions into the following two categories: “HPV-associated squamous intraepithelial lesions” and “HPV independent VIN” (Table 3). The subtypes of HPV-independent VIN include differentiated exophytic vulvar intraepithelial lesions, vulvar acanthosis with altered differentiation, and dVIN.
Epidemiology
The LSILs of vulvar condyloma are typically associated with low-risk HPV infections (predominantly HPV 6 or 11 in 90% of cases) [17]. Occurring at a frequency of approximately 107–229 per 100,000 women, LSILs are widespread in the general population and do not progress to invasive malignancies [18,19]. Conversely, vulvar high-grade squamous intraepithelial lesions (VHSIL), which are 2.5–8.8 times more prevalent than LSIL per 100,000 women annually, have the potential to progress to invasive cancers [17,20,21].
Notably, dVIN, constituting less than 10% of squamous vulvar intraepithelial lesions, has a higher risk of malignant transformation than VHSIL (32.8% in older women with dVIN vs. 5.7% in younger patients with VHSIL) [22,23].
Patients with VHSILs have a greater risk of anal squamous cell carcinoma and its precursors because of the HPV field infection, even though anal cancer is rare in the general population (1–2 incidences per 100,000 person-years).
According to a recent meta-analysis, women with VHSIL have the third-highest incidence ratio of anal cancer, reaching 42 per 100,000 person-years (95% confidence interval, 33–52), following human immunodeficiency virus (HIV)-positive men who have sex with men aged ≥30 years old and transplanted women ≥10 years post-transplant. The mean time interval between the incidence of VIN and anal cancer diagnosis was reported to be 8.9 years [24,25].
Molecular biology
The etiology of VHSIL, the precursor of HPV-related invasive cancer, involves high-risk HPVs (HPV 16 in >70% of cases), smoking, and immunosuppression [26,27]. The oncogenesis of VHSIL is similar to that of cervical, vaginal, and anal HSIL. Molecular heterogeneity has been observed among the anogenital HSILs. When considering conservative care for VHSIL, significant levels of host cell DNA methylation appear to indicate a high risk for malignancy [28]. A study using whole-genome shallow sequencing revealed that a gain in chromosome 1pq serves as a powerful predictor of the likelihood of HPV-positive VIN developing into vulvar squamous cell cancer [29].
Chronic inflammatory lymphocyte-mediated skin disorders, such as LS or lichen planus, are the primary causes of dVIN and HPV-negative vulvar squamous cell carcinoma [30].
TP53 mutations are typically observed in patients with dVIN. Similar to HPV-negative vulvar squamous cell carcinoma, clonal D1 amplification and copy number alterations in chromosomes 3, 8, and 11q13 have been documented in HPV-negative VIN [29,31].
A third, as yet unidentified molecular subtype has been suggested based on the discovery that a portion of HPV-independent precursors are TP53 wild-type with somatic mutations in PIK3CA, NOTCH1, and HRAS [32–34]. According to proteomics studies, inflammation plays a key role in the course of LS and lichen planus, where chronic inflammatory conditions are thought to be the primary causes of oxidative damage and local immunological dysregulation. Disturbances in the vulvovaginal microbiome also appear to cause inflammatory response, which modifies the balance of commensal microorganisms in the host [35–37].
Clinical features
Clinical features crucial for making a correct diagnosis are color, thickness, surface characteristics, and focality. Approximately 60% of patients experience symptoms [20,38].
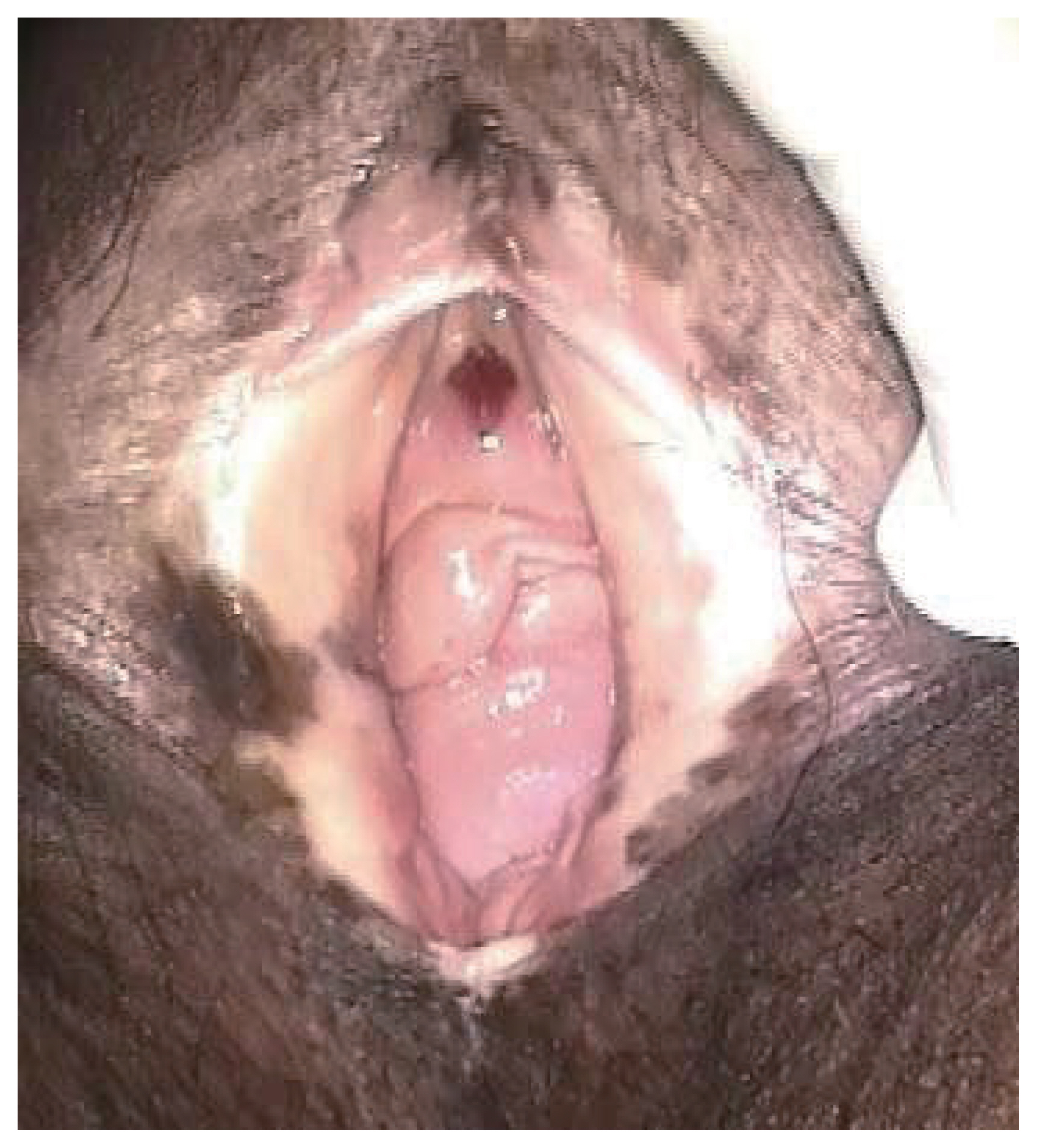
To confirm the diagnosis, a biopsy of the most suspicious part of the lesion should be performed under local anesthesia [39]. The commonly affected sites are the labia majora and minora, as well as the fourchette [40]. Lesions can be red, white, or pigmented; flat or raised; with the presence of erosions or ulcers (Fig. 1). Differentiating between different forms of vulvar lesions based solely on macroscopic features and the distribution of vulvar alterations is challenging due to notable variations in the number, size, form, color, surface characteristic, thickness, and topography of vulvar squamous intraepithelial lesions. One or more lesions may be present, with keratotic, roughened surface, sharp edges and a papular, elevated appearance displaying white, red, gray, blue, or brown colors. After a thorough inspection with the unaided eye, magnification of the vulvar skin with a lens or colposcope may enable (A) a better characterization of the extent of the lesion; (B) guidance for biopsies to the area of the most clinically severe abnormalities; and (C) visualization of anatomic landmarks to guide treatment. When HPV-associated squamous intraepithelial lesion is suspected, the application of 3–5% acetic acid by skilled practitioners may reveal an elevated and finely delineated acetowhite epithelium, typically corresponding to VHSIL (Fig. 2); however, dVIN typically does not react to acetic acid. Because of the high false-positive rate in vulvoscopy, acetic acid should only be used by skilled practitioners [41]. Young women are more likely to develop VHSIL, which are typically multifocal, centered on the introitus, and frequently involve the labia minora. Multicentric/multizonal illnesses frequently manifest in women with VHSILs and can affect the squamous epithelium of the cervical, vaginal, perianal, or anal regions. A thorough examination of the vulva, perineum, perianal, and anal regions, including the cervix and vagina, is essential. High-resolution anoscopy screening for all patients with VHSILs is not feasible, and anal cytology sensitivity appears to be low in women [42]. It is sometimes challenging to differentiate dVIN from related dermatosis, especially when LS affects the nearby skin. dVIN typically manifests as weakly defined, unifocal, and unicentric rough plaques that are pink or gray-white (hyperkeratotic) [43,44]. To exclude dVIN, a lesion biopsy should be performed in cases of persistent symptoms and dermatoses that do not respond to treatment. Up to 20% of patients with VHSILs may have an underlying early invasive squamous carcinoma; this number is even greater in patients with dVIN. A biopsy must be performed to provide a firm diagnosis of vulvar lesions. Since many vulvar malignancies go undetected and are diagnosed later because biopsies are not obtained, it is important to perform a biopsy on any suspicious lesion, and repeated biopsies should be performed for large, multicentric, and multicolored lesions. The diagnosis is established using a punch or incision biopsy, and each lesion should be mapped and biopsied independently.
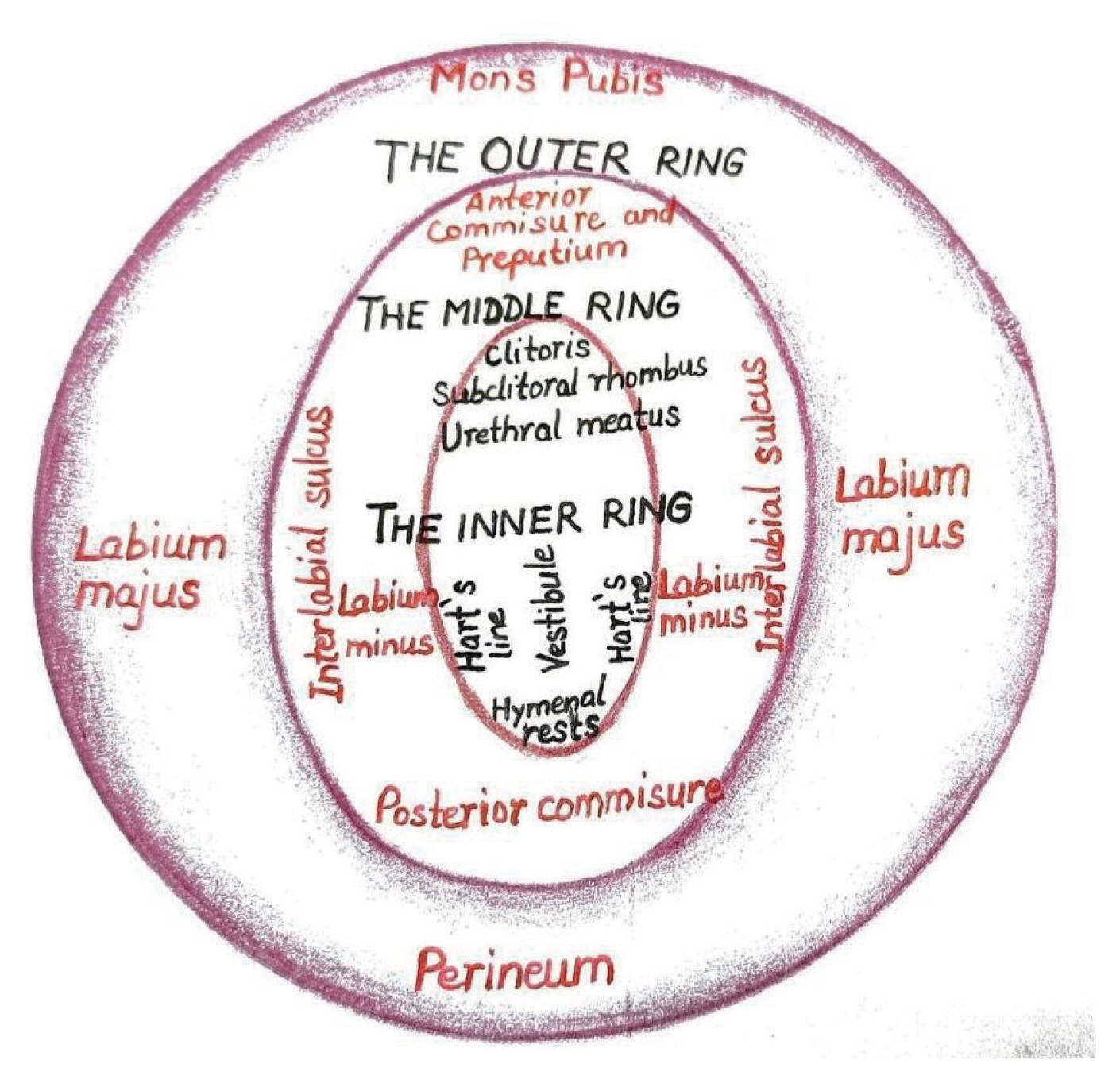
Three ring vulvoscopy
Understanding the histology of vulvar skin is crucial when performing a colposcopic examination of the vulva because the intricate architecture of this region necessitates a distinct evaluation of lesions that appear to be similar in nature. Opacity is influenced by the thickness of the vulvar skin, and unlike cervical colposcopy, vascular patterns are less pronounced and less dependable. The vestibular epithelium lacks a keratin layer; therefore, vascular aberrations such as punctuation and mosaics are only visible on the inner parts of the labia minora, where the keratin layer is thinner [45]. A novel approach to vulvoscopy has been suggested that accounts for three distinct skin types and nearly ring-shaped zones. This is a circular, designed vulva observation, henceforth referred to as “three rings vulvoscopy”, as opposed to a random or linear vulvoscopy. The description of the outer, middle, and inner vulvar rings is based on vulvar histology and embryology (Fig. 3).
The term outer vulvar ring refers to vulvar skin derived from the ectoderm and is the natural outer boundary of the vulva. It is comprises hair-bearing; keratinized skin containing sebaceous, apocrine, and eccrine glands; subcutaneous fat; and blood vessels [46]. This encompasses the perineum, labia majora, and mons pubis.
The middle vulvar ring refers to the modified mucosa of ectodermal origin that functions as an intermediary circuit between the vestibule and labia majora. It has nonhair-bearing skin all over it, with sebaceous glands but lacking subcutaneous fat. This comprises the labia minora, fourchette, prepuce, frenulum of the clitoris, and the anterior commissure.
The inner vulvar ring is a glycogenated squamous mucosa of the non-keratinized type, non-pigmented stratified squamous epithelium, entirely devoid of skin appendages, except for a small region in front of the urethra. It encompasses the urethral meatus, hymenal remnants, Bartholin’s gland entrance, Hart’s line, clitoris, subclitoral rhombus (also known as the sulcus urethralis), and the vestibule. The vestibular line of Hart, which connects the keratinized and non-keratinized epithelia on the inner sides of the labia minora, demarcates the inner and middle rings.
Together with the vulva, the lower genital tract comprises the anus, perianal area, and groins. Because the skin of the groin and perianal regions is made of the same tissue as the skin of the outer ring of the vulva, the lesions can be described similarly.
Vulval skin pathologies
For managing patients presenting with vulvar symptoms, a systematic approach is essential. It is crucial to recognize that vulval itch, often termed “pruritus vulvae”, is a prevalent complaint, yet it serves as a symptom rather than a standalone diagnosis, indicating an underlying cause. This itching can be the initial manifestation of various vulval skin disorders or may be linked to a broader systemic ailment.
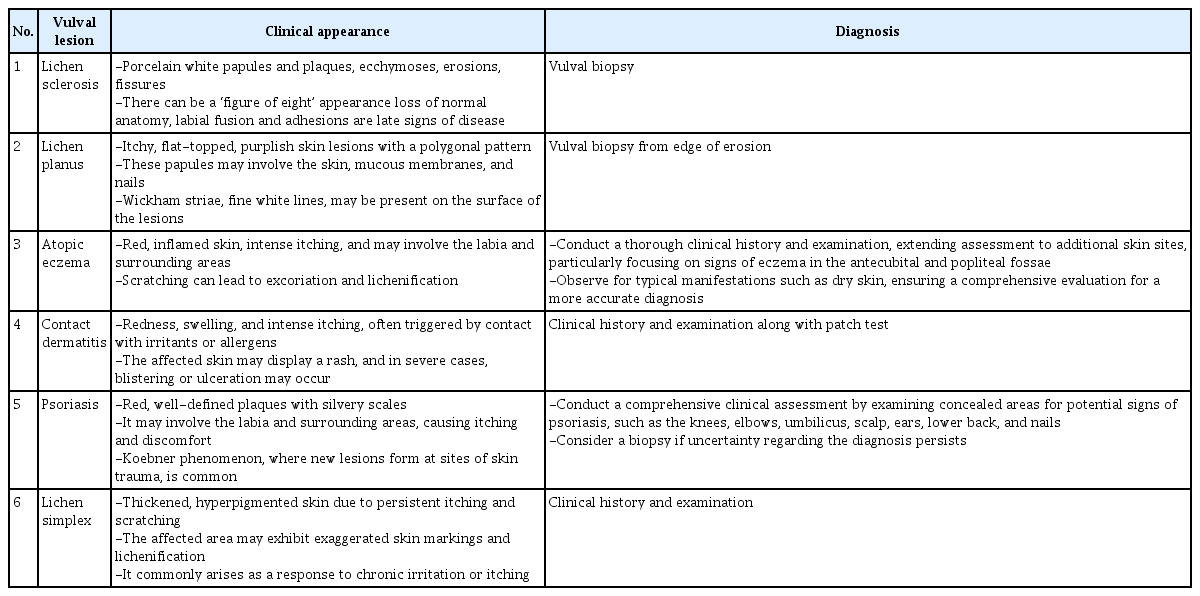
Common dermatoses affecting the anogenital area, which can affect any female, include dermatitis (irritants, allergic contact, or atopic dermatitis), psoriasis, and LS. Additionally, an array of local factors may contribute to anogenital itching, including infections, such as candidiasis, viral warts, urinary or fecal incontinence, lichen simplex chronicus, squamous cell carcinoma, and estrogen deficiency. Acknowledging these potential causes is pivotal for a comprehensive and accurate diagnosis, allowing for a targeted and effective management approach tailored to specific underlying conditions. Table 4 shows the clinical appearance and diagnosis of benign vulvar dermatoses.
Histopathology
Pathologists working with high-volume vulvar samples must accurately histologically diagnose vulvar intraepithelial lesions to determine the best course of action. For tissue samples of suspected precursor lesions, it is advised to use a punch, cold knife, or suture-assisted snip to collect ideal specimens with a minimum width of 4 mm and a depth of 5 mm for hair-bearing skin and 3 mm for hairless skin and mucosal areas. Where the epithelium is intact, a biopsy should be performed in for an ulcer or fissure [47]. Immunohistochemistry is useful for differentiating challenging cases from non-invasive vulvar lesions. The histological features of dVIN can be subtle, and the histological diagnosis may be further complicated by co-existing conditions, such as LS. According to van de Nieuwenhof et al. [39], 42% of biopsies that were initially diagnosed as LS were reclassified as dVIN after a review. VLSIL exhibits abnormal maturation and dysplastic features up to the lower third of the epithelium (Fig. 4), and these abnormal features extend above the lower third of the epithelium. Immunohistochemistry with p16 can be helpful in differentiating VLSIL from VHSIL (Figs. 5, 6) or atrophy from VHSIL. Basal atypia in the dVIN is characterized by parakeratosis, basal spongiosis, a lack of a granular layer, and abrupt (premature) maturation (hypereosinophilic keratinocytes) (Table 5).
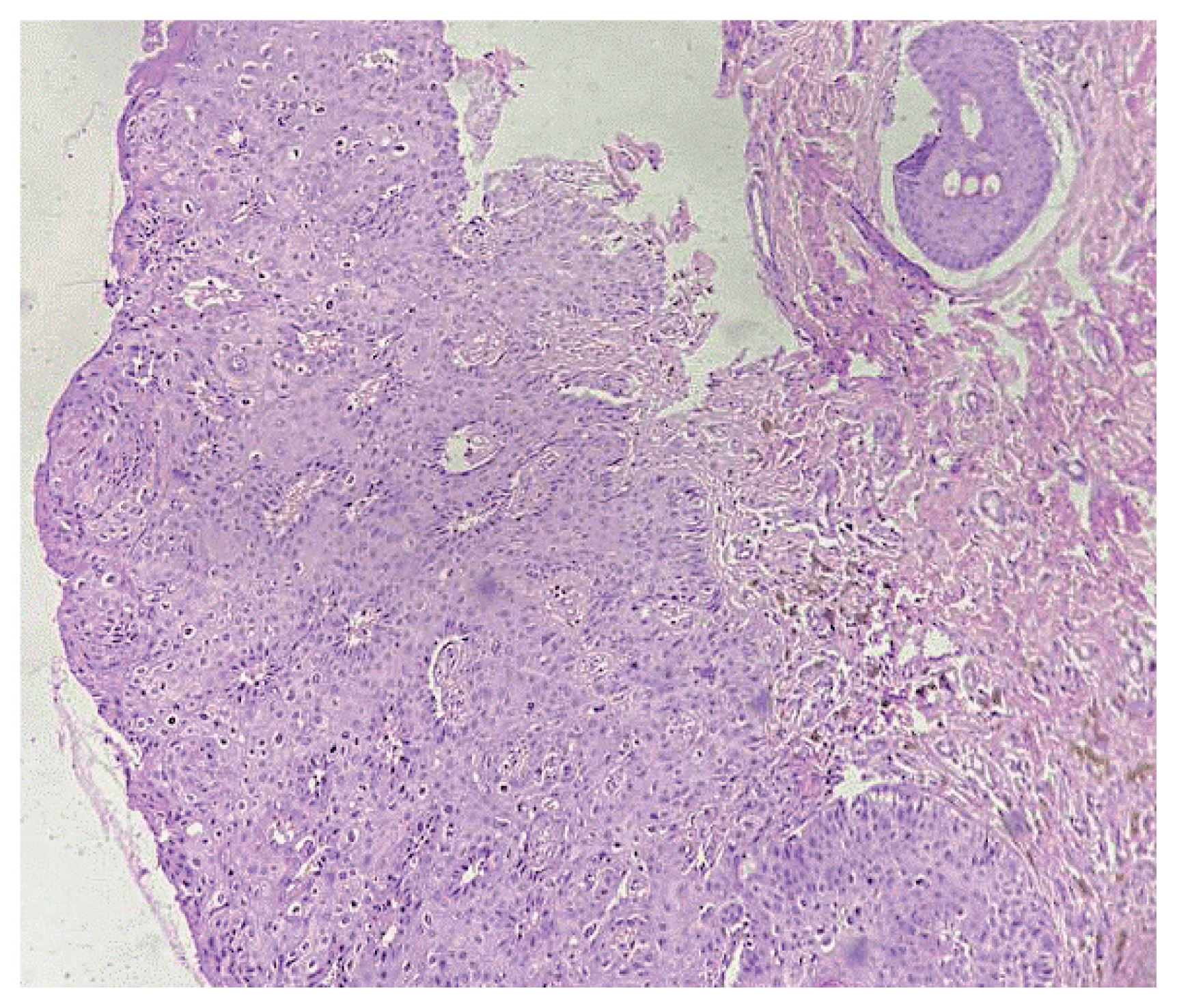
Histological sections reveal acanthosis, along with atypical koilocytosis in the upper layers. Mild atypia and mitotic activity limited to the lower third of the epithelium are suggestive of VIN 1 (×100 magnification). VIN, vular intraepithelial neoplasia.

(A) Histological sections reveal acanthosis with nuclear atypia and increased mitotic activity involving the lower two-thirds of the epithelium, suggestive of VIN 2 (×400 magnification). (B) Immunohistochemistry reveals block positivity for p16 (×400 magnification). VIN, vulvar ntraepthelial neoplasia.

(A) Histopathological sections reveal acanthosis with loss of polarity, full thickness nuclear atypia, and increased mitotic activity, suggestive of VIN 3 (×400 magnification). (B) Immunohistochemistry reveals block positivity for p16 (×400 magnification). VIN, vulvar ntraeithelial neoplasia.

Immunohistochemical distinction between vulvar high-grade squamous intraepithelial lesions (VHSIL) and differentiated vulvular intraepithelial lesions (dVIN)
Premature keratinization with hypereosinophilic keratinocytes and nuclear atypia, including larger and angulated hyperchromatic nuclei and enhanced mitotic activity, may be observed.
Squamous hyperplasia, rete ridge elongation, prominent intercellular bridges in the lower epithelium, and the lack of a granular layer in conjunction with hyperkeratosis and parakeratosis are common characteristics of dVIN [48]. p53 frequently exhibits an abnormal staining pattern in dVIN dysplastic cells.
Immunology
A local immunosuppressive milieu with increased T-regulatory cell infiltration, increased CD4+ (T helper cells) infiltration, and decreased CD8+ (cytotoxic T cells) count can be induced by persistent HPV infection in VHSIL [49,50]. Patients with non-recurrent and recurrent VHSIL were investigated for the presence and clinical significance of several myeloid cell types, and the non-responding group exhibited the highest intraepithelial CD14+ (a monocyte marker) count. The population of M2 macrophages in VHSIL was at least four times greater than that of M1 macrophages, indicating an immunosuppressive environment within the VHSIL epithelium [51]. Numerous regulatory T cells (Tregs) have invaded certain VHSIL lesions, creating an immunosuppressive milieu [52]. In VHSIL, a decrease in Treg counts and an increase in intralesional CD8+ and T cells are linked to the clinical response to immunotherapy. It is true that the histological regression of VHSIL is associated with the normalization of CD4+, CD8+, and T cells counts in the epidermis and the elimination of HPV [49]. A decrease in intraepithelial CD14+ and cells and an increase in CD1a+ and langerhans cells were linked to HPV clearance during imiquimod therapy for VHSIL [49]. An independent predictor of reduced recurrence-free survival and an increase in CD14+ and myeloid cells indicates a progressive course of vulvar neoplasia [53].
Management
Adopting a holistic approach for all vulval skin conditions, emphasizing patient education, support, and counseling. Information should be provided through leaflets, websites, and written instructions. The use of mirrors or models in clinics helps guide topical treatment applications. Addressing skin or mucosal barrier breakdown in vulval conditions involves avoiding irritants, such as soap, and employing soap-substituting emollients. Irritation due to urinary and fecal incontinence should be managed to prevent worsening of the underlying skin pathology. The use of bland emollients must be emphasized for cleansing and moisturizing, tailored to patient preferences. Topical steroids should be used to reduce inflammation in conditions, such as lichen planus, LS, and eczema, thereby improving symptoms and appearance. Concerns about side effects must be alleviated by ensuring correct steroid strength, duration of application, and application site. This emphasizes that mucosal surfaces, such as the vulval vestibule, are resistant to steroid atrophy when applied correctly.
Vulvar squamous intraepithelial lesions
Excisional surgery is typically necessary for dVIN. Both excisional and ablative techniques can be applied in VHSIL. The latter can be considered to preserve anatomy and function, but in order to rule out malignancy, multiple representative biopsies must be performed first. Medical therapy (imiquimod or cidofovir) may be considered for VHSILs.
In the past, the usual course of treatment involved a major surgery to completely treat this condition. However, current goals focus on maintaining the quality of life and sexual function with tailored treatments, avoiding progression to vulvar squamous cell carcinoma, preserving normal anatomy, and relieving symptoms. A long-term follow-up study revealed variation in the median cancer progression time after VIN diagnosis, ranging from 0.3 to 24.2 years (1.4 years for dVIN and 4.1 years for VHSIL).
According to a 2016 Cochrane analysis, 15% of women receiving surgical treatment for VHSIL over a median of 71.5 months experience a progression to squamous cell carcinoma [54].
Treatment with high-potency topical corticosteroids reduces the risk of vulvar squamous cell cancer in LS (via a dVIN route) and should be recommended in these patients [55–58].
Surgical interventions
The only available treatment for dVIN is conservative excision with negative surgical margins and ongoing follow-up due to the short-term risk of developing invasive vulvar squamous cell carcinoma [59,60]. Medical treatment using dVIN ablation is not recommended.
Both surgical excision (ranging from superficial vulvectomy to wide local excision) and ablative therapy (argon beam coagulation, carbon dioxide [CO2] laser vaporization, and cavitational ultrasonic surgical aspiration) are options for treating VHSIL. The latter treatment must be chosen with representative biopsies performed beforehand to rule out cancers because of the risk of unanticipated stromal invasion. If a clinical examination reveals no residual lesion in the case of positive margins following surgical excisional therapy for VHSIL, patients should be monitored; prompt re-excision is not advised in such cases. Considerably impaired surgeries should be avoided and extensive resections should only be performed under the supervision of competent reconstructive surgeons when necessary.
Despite treatment, the rate of VIN recurrence varies from 6% to 50% after treatment [61–65]. Factors related to the patient, such as smoking, immunosuppression, and multiple focalities of the disease, as well as the type of VIN (even though the exact differences in disease outcomes between VHSIL and dVIN are not always clear) affect this rate. Within 16.9 months, 50% recurrences had been reported, necessitating careful monitoring in the first 2 years following surgery, especially in patients aged >50 years. According to Leufflen et al. [66], recurrence-free survival at 1 year was 91.0% in the surgical group and 65.2% in the laser vaporization group (P<0.01). After receiving either treatment, the mean time until recurrence was 21.7 months. At an average follow-up of 4.4 years (range, 0.8–18.4), 2% of patients progressed to invasive illness.
According to van Esch et al. [67], women who underwent surgery had a lower recurrence rate (48.8%) than that of patients who underwent laser ablation (56.0%) or combined laser and excision (66.7%). Additionally, Wallbillich et al. [68] reported a greater recurrence rate associated with laser ablation (45%) than with cold knife excision (26.7%).
The efficacy of argon beam coagulation was assessed for treating VIN3 (VHSIL); the mean time to recurrence was 23.2 months, and the recurrence rate was 48.3%. Preserving vulvar anatomy and facilitating repeated treatments are the key benefits of this therapeutic approach.
In a single randomized controlled study, cavitational ultrasonic aspiration (CUSA) and CO2 laser vaporization were compared. At the 12-month follow-up, no statistically significant difference in recurrence was observed between them, and CUSA was found to cause less discomfort and scarring than the laser.
A recurrence rate of 35% after a median interval of 16 months and a progression rate of 3% after a median follow-up of 33 months were observed for treating VIN using CUSA alone [69].
Medical interventions
For VHSIL, medical therapy is a viable therapeutic option to prevent mutilation and maintain normal vulvar anatomy. However, the risk of histological specimens lacking early invasive foci is not present with the medicinal therapy. Consequently, multiple biopsies are required prior to medical treatment.
Imiquimod is an immune response modulator that targets TLR-7 and induces significant immunological infiltration by stimulating the release of pro-inflammatory cytokines by dendritic cells [70,71]. Two randomized controlled trials compared imiquimod with a placebo after 87% of patients who participated in a pilot study experienced a complete or partial response.
Between 2 and 5 months after treatment, Mathiesen et al. [72] and van Seters et al. [73] reported a complete response rate of 81% and 35%, respectively, among women treated with imiquimod. In a 12-month follow up period, van Seters et al. [73] found no difference in the rates of progression to invasive illness between the two groups (1/26 vs. 2/26), with 35% of complete responders (n=9) in the imiquimod group compared with 0% in the placebo group. After a median follow-up time of 7.2 years, eight of the nine initial complete responders remained disease-free. Patients with persistent and/or recurrent illnesses had considerably larger lesion diameters than those of the long-term full imiquimod responders.
In a randomized controlled trial including 180 patients, the researchers observed no difference in complete response rate (46% for both groups) between topical 5% imiquimod cream and 1% cidofovir gel [74]. In another study, 87% of complete responders to cidofovir and 78% of complete responders to imiquimod continued to show positive results at the 12-month follow-up. The complete responders to cidofovir exhibited a 6% recurrence rate after an 18-month, compared with 28.4% observed for complete responders to imiquimod [75].
HPV E2 DNA methylation has been shown to be a predictive biomarker for a good response to cidofovir treatment in VINs [76]. A 20.5–27.0% recurrence was reported after 16–21 months of follow-up in two further non-randomized controlled trials of imiquimod as a single therapy [77,78].
Cold-knife surgery plus imiquimod cream as an adjuvant may allow for less extensive excision and greater preservation of the anatomy and function; however, it does not appear to offer advantages in terms of a decreased recurrence rate.
Photodynamic therapy
To induce oxidation events that result in cell death, photodynamic therapy combines nonthermal light of the proper wavelength with a topical photosensitizer, 5-aminolevulinic acid. The overall clinical response is similar to that of laser ablation [79], ranging from 31.2% to 56% [80]. The recurrence rate varies between 14.3% at an average follow-up of 13 months and 48% at an average follow-up of 53.7 months. According to a study, the rate of invasion following therapy was 9.4%.
Therapeutic vaccine
Investigations into therapeutic vaccines against HPV-16 E6 and E7 oncoproteins have yielded encouraging findings in an observational phase II study, with 47% of the patients exhibiting a complete response and 32% exhibiting a partial response at the 12-month follow-up; patients who exhibited a complete response remained disease-free at the 24-month follow-up.
Follow-up of women with vulvar intraepithelial neoplasia
After receiving treatment for VIN, women should be scheduled for routine visits for a thorough clinical evaluation, including a biopsy of any suspicious area. The frequency of follow-up appointments should be adjusted based on the lesion type, patient age, immunological status, and related lower genital tract lesions, taking into account the likelihood of recurrence.
Reports indicate a broad range of risks for progression to malignancy, estimated to be 10% for VHSIL and up to 50% for dVIN. Despite surgical treatment for VIN, women still bear a residual 2–4% risk of developing invasive cancer. Regardless of the surgical technique, there is a 60% chance of VIN recurrence.
Providing clear information about symptoms and indicators (discomfort or ulcers) is crucial for prompting an early review by women. Although the risks of invasion and long-term clinical results after topical medication therapies that achieves a full clinical response are not well established, they may resemble surgical treatment.
Approximately 4% (up to 25%) of women with VIN develop intraepithelial neoplasia at other lower genital tract sites [81,82]. During follow-up, it is imperative to accurately evaluate all lower genital tract sites, including the cervix, vagina, and vulvar and perianal skin. In one study, the incidence of VHSIL was similar regardless of whether the woman had undergone a prior hysterectomy, suggesting that vaginal surveillance is still necessary.
Initiatives for screening vulvar squamous cell carcinoma and VIN associated with HPV infections should be launched to enhance early detection and management.
Prevention
The majority of vulvar LSIL and VHSIL are linked to HPV; the most common HPV types in LSIL, VHSIL, and HPV-related invasive vulvar cancers are HPV 16 and 33, and HPV 6 and 11 in LSIL [83]. HPV vaccinations are highly effective in preventing lesions associated with various vaccine types [84,85]. Nonvalent HPV vaccine-associated HPV genotypes are associated with more than 90% of these lesions. Women with vulvar diseases linked to HPV infection are highly susceptible to developing subsequent or recurring illnesses.
The incidence rate of 8.1:1,000 person-years in women with LS indicated a 3.5% risk of cancer, and this risk increases with age [86,87]. Compliant women with lichen sclerosis who were treated with topical steroids showed improved symptom control and a significantly lower risk of vulvar carcinoma.
Current recommendations include advising women to continue using topical steroids on a weekly basis, even if they are asymptomatic, and to undergo routine checkups for the rest of their lives (at least every 6–12 months or whenever new lesions are discovered or symptoms do not improve with appropriate therapy). Patients under control can schedule follow-up appointments with their primary care physicians. Any worrisome lesions (tumors, chronic erosions, or hyperkeratosis) or lesions that do not respond to treatment should be biopsied. Following cancer therapy, topical steroids are rarely administered to women with vulvar cancer and lichen sclerosis; however, if used, the chance of recurrence can be reduced by half (27% vs. 44–47%).
Immunosuppressed patients
In addition to women receiving immunosuppressive therapy for autoimmune or rheumatological disorders, those with HIV comprise the immunosuppressed population. Evidence suggests that immunosuppression increases the risk of developing invasive malignancies and preinvasive lesions associated with HPV.
HIV disrupts epithelial tight junctions, making HPV infections easier to follow. HIV and HPV have close immunological interactions. Furthermore, immune system abnormalities, including a decrease in CD4+ lymphocytes, may impede the elimination of latent HPV infections and cause reactivation [88,89].
In addition to multifocal and multicentric HPV-related lesions, women with HIV have greater incidence rates of VIN at younger ages. Although it did not seem to have any effect on VHSIL, highly active antiretroviral therapy may reduce the incidence of condyloma and LSIL.
Immunosuppressive medications may increase the risk of HPV carcinogenesis in recipients of kidney transplants. Within 20 years of transplantation, recipients of renal transplants have a greater chance of developing VHSIL (5–12%) than that of recipients of non-renal transplants (0.2–0.4%) [90].
Additionally, a Dutch study reported a 122-fold increased risk of anal cancer and a 41-fold increased risk of vulvar cancer in recipients of renal transplant. Remarkably, in this cohort, all cases of vulvar cancer were positive for HPV; however, in immunocompetent patients, the percentage was as low as 4.9% [91–94].
Therefore, as part of the routine screening, immunosuppressed individuals should undergo a thorough inspection of their lower genital tract.
Quality of life and psychological sequelae of vulvar preinvasive lesion treatment
Preinvasive vulvar lesions are particularly important because they affect psychosexual variables, functionality, and body image. Dyspareunia and feelings of decreased attractiveness may result from burning and itching associated with intraepithelial neoplasia, as well as a change in the appearance of the vulvar skin. The emotional load may also be increased by concerns about contaminating the partner with HPV-related VIN and the possible consequences for a subsequent pregnancy. Because the presence of scars from surgery and the fear of exposing their bodies may exacerbate sexual dysfunction rather than improving it. Women often fear that their cancer may return or spread. Those with VIN generally have a lower quality of life. Couples counseling combined with partner education and psychological support from gynecologists, psychologists, or psychiatrists may help regaining sexual confidence, restoring sexual functioning, and increasing quality of life.
Conclusion
The prevalence of VIN is on the rise, especially in women in their 40s. Although VIN is a premalignant condition, cases of spontaneous regression have been reported. Immunization with the quadrivalent or 9-valent HPV vaccine reduces the risk of VHSIL (uVIN) in girls, aged 11 and 12 years, with catch-up vaccinations recommended until 26 years of age for those not vaccinated at the target age. The vaccine is effective against HPV genotypes 6, 11, 16, and 18, as well as 6, 11, 16, 18, 31, 33, 45, 52, and 58. Currently, no screening methods are available for the early detection and prevention of vulvar cancer VHSIL (uVIN). Histopathology is used only when o confirming the visual assessment-based detection is necessary. Treatment is recommended for all women with VHSIL (uVIN). Wide local excision should be performed if cancer is suspected, even if biopsies reveal VHSIL, owing to the possibility of undetected invasion. Topical imiquimod, laser ablation, and excision are treatment options for vulvar HSIL (typical VIN-type) when occult invasion is not a concern. Women who respond completely to treatment and do not develop new lesions at follow-up appointments at 6 and 12 months following treatment initiation should have their vulva visually inspected annually thereafter.
Notes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Waived due to literature review.
Patient consent
Waived due to literature review.
Funding information
None.