Validity of ultrasound with color Doppler to differentiate between benign and malignant ovarian tumours
Article information
Abstract
Objective
To assess the utility of ultrasound and color Doppler and the Accuracy of International Ovarian Tumor Analysis (IOTA) group classification in the preoperative evaluation of ovarian neoplasms to assess benign or malignant histopathology in the diagnosis of ovarian tumors.
Methods
This observational longitudinal prospective analysis of 60 patients was performed over a period of 2 years (2017–2019). The mean age of the patients was 43.75 years. Ultrasonography of ovarian masses were evaluated, and cancer antigen-125 (CA-125) levels were evaluated. Based on the IOTA classification, the B and M features of adnexal masses were studied. Color Doppler imaging was performed to evaluate the patterns of vascularity and indices.
Results
Sixty patients with 35 benign, 23 malignant, and two borderline lesions were included in the study. In malignant lesions, 17 women (73.9%) were above the age of 45. The CA-125 cut off was ≥35 internatioal units/mL. Based on the IOTA classification, 27/35 (77.1%) benign cases, were correctly identified as benign, 6/35 (17.1%) benign cases were incorrectly identified as malignant, and two (5.7%) were found to be inconclusive. In the malignant group, 17 of the 23 patients were identified as having malignancy. Color Doppler showed three (18.8%) benign tumors had a pulsatality index (PI) of <0.8 and 21 malignant tumors had a PI of <0.8. Four benign tumors had an resistive index (RI) of <0.6 and 100% of malignant tumors had an RI <0.6.
Conclusion
The IOTA classification is a reliable scoring system for adnexal masses, and color Doppler can help to minimize interobserver variation.
Introduction
The life time risk of developing ovarian cancer is approximately 1.3%. The 5-year survival rate of ovarian cancer decreases from 92.3% in cases confined to the ovary to 29.2% in those with distant metastasis. Almost 59% of women with ovarian cancer are diagnosed after metastases have already developed [1,2]. Preoperative assessment of the risk of malignancy in an adnexal tumor can be performed using several methods. These include a pattern recognition approach, International Ovarian Tumor Analysis (IOTA) group mathematical logistical regression models, simple ultrasound-based rules for the diagnosis of ovarian cancer, risk of malignancy index models, and Gynecological Imaging Reporting and Data systems.
A pattern recognition approach or subjective assessment of an ovarian tumor based on sonographic morphology and Doppler assessment of vascularity remains the gold standard to assess the preoperative risk of malignancy assessment. In the pattern recognition approach, ovarian tumors can be classified into one of five groups: unilocular cysts, unilocular solid cysts, multilocular cysts, multilocular solid cysts, or solid tumors [3].
The IOTA group developed a logistic regression model to preoperatively assign a risk of malignancy to ovarian tumors in patients undergoing surgery. This includes a standardized methodology for the ultrasound evaluation of ovarian masses and definitions of the ultrasonographic parameters of ovarian masses [4]. To ensure correct assessment, the entire tumor must be examined using both color and spectral Doppler imaging. A score can be assigned based on the amount of vascularity, as follows: 1, no flow: 2, minimal flow: 3, moderate flow: and 4, highly vascularized mass, as per the ovarian adnexal imaging reporting data system classification. Spectral Doppler assessments of the highest time-averaged maximum velocity, corresponding peak systolic velocity, pulsatility index (PI), and resistive index (RI) were performed [5].
Past studies found that 23% of ovarian cancers were related to hereditary conditions [6]. Advances in genetic analyses have enabled the detection and characterization of the genetic types involved in the pathogenesis of ovarian neoplasms. Germline and somatic mutations can be seen in both the hereditary and sporadic forms of Sertoli-Leydig cell tumors, and the hypercalcemic type of small cell ovarian carcinomas shows biallelic inactivating mutations of associated genes [7].
The aim of this study was to differentiate between benign and malignant ovarian masses on the basis of ultrasound and color Doppler findings and IOTA classification, and to validate the findings by correlation with histopathology.
Materials and methods
1. Study location
This study observational longitudinal prospective analysis of 60 patients admitted to a tertiary care hospital over a period of 2 years (September 2017 to August 2019) was performed at Government Lady Goschen Hospital and Mangalore and KMC Hospital, Attavar, Mangalore, India. All patients underwent evaluation and operative intervention for ovarian masses. The exclusion criteria were as follows: 1) pregnant women; 2) masses with infectious pathologies; 3) patients with endometriosis; 4) pelvic masses other than those of adnexal/ovarian origin; and 5) patients who did not undergo surgical treatment or were unfit for surgery.
The Simple Rules, as proposed by the IOTA group, comprise five features typical of benign tumors (B-features) and five features typical of malignant tumors (M-features) (Table 1, Fig. 1). These Simple Rules suggest that an adnexal mass can be malignant, benign, or inconclusive based on the presence or absence of M- and B-features (Table 2, Fig. 2). In this study, the IOTA ADNEX model calculator was used to accurately predict benign versus malignant tumors. Vascularity was investigated based on its presence/absence, and location (peripheral/central), with a PI cut of 0.8 and RI cut off of 0.6.

The simple rules proposed by the IOTA group comprise five features typical for benign tumours (B-features) and five features typical for malignant tumours (M-features)
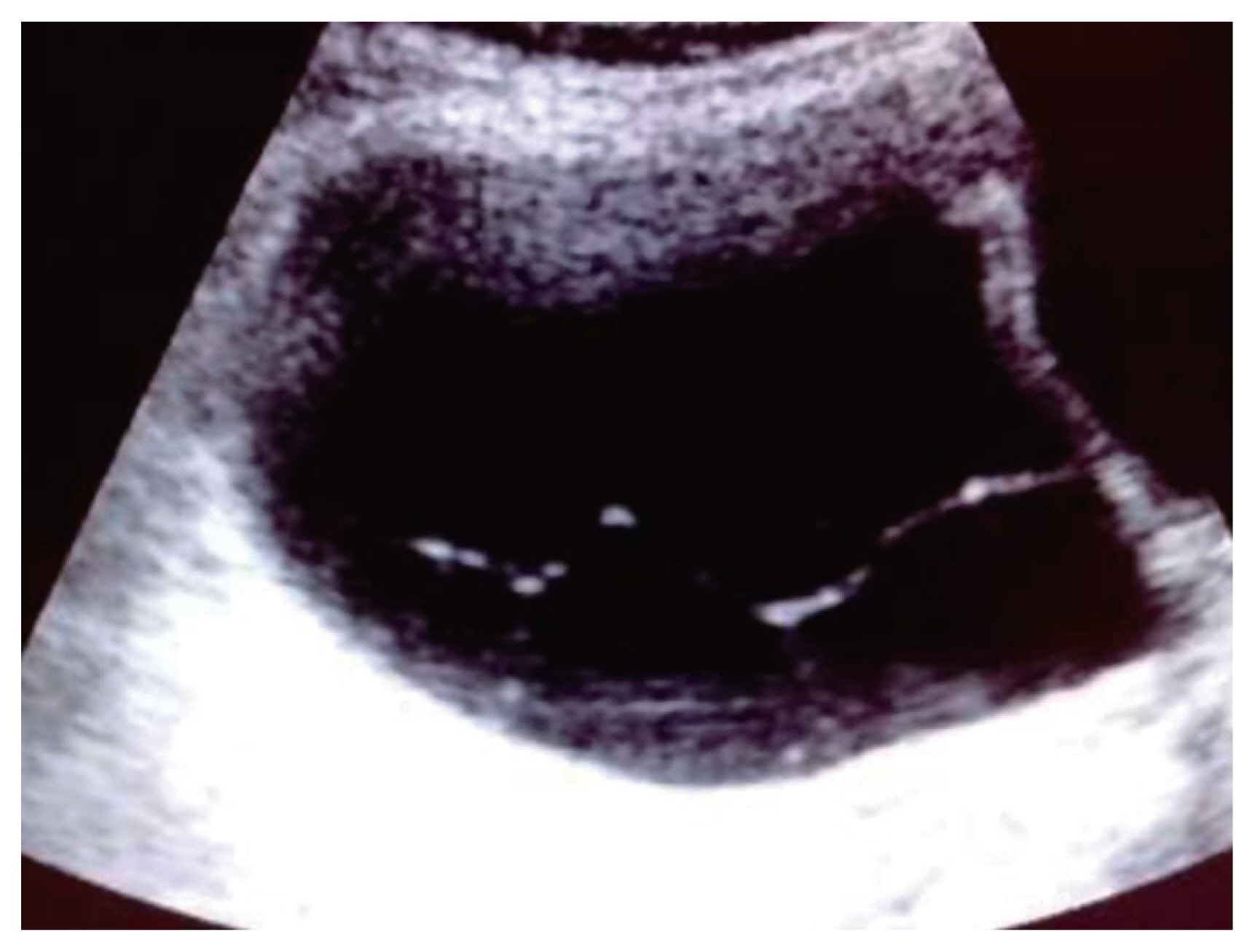
Serous cystadenoma with fine internal septae (<3 mm) and benign sonological features according to the IOTA classification. IOTA, International Ovarian Tumor Analysis.
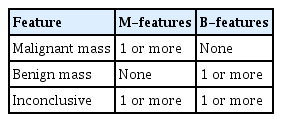
The simple rules propose that an adnexal mass can be malignant, benign or inconclusive based on the presence or absence of M- and B-features
2. Statistical analysis
The collected data were coded and entered into Statistical Package for the Social Sciences SPSS 11.5 (IBM, Chicago, IL, USA). Descriptive analyses were performed for all variables to determine the distribution of categorical variables and mean values of continuous variables. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated for the two diagnostic modalities. The student’s t-test and chi-square test were used for comparisons across groups. Statistical significance was set at P<0.05 significant.
Ethical approval was obtained from the committee (Institutional Ethics Committee of Kasturba Medical College, Mangalore Region. No.ECR/541/Inst/KA/2014, IEC KMC MLR 08-17/144), and informed consent was obtained from all patients prior to inclusion in their study.
Results
A total of 60 patients were included in this study, of whom 35 were benign, 23 were malignant, and two were borderline. The mean age of the patient cohort was 43.75 years (range, 12–75). Of the 23 women with malignant masses, 17 (73.9%) were above the age of 45 years, while the remaining six (26.1%) were aged between 20–45 years. Conversely, in the benign group, 20 patients were aged less than 45 years of age and only 15 (42.9%) cases were >45 years of age. In the borderline group, one patient was 18 years old, while the other was 23 years old (Table 3).
The cut-off value for cancer antigen 125 (CA-125) over which a mass was considered as malignant was ≥35 international units (IU)/mL. The mean CA-125 values in the benign and malignant groups were 38.00 and 730.41 IU/mL, respectively. Evaluation of the CA-125 levels revealed that 25 (71.4%) benign cases had a CA-125 <35 and 10 (28.6%) benign cases had CA-125 ≥35, whereas in the malignant group, 18 (78.3%) cases had an elevated CA-125 ≥35, representing a highly significant difference (P<0.001) (Table 3).
Regarding the size of the adnexal mass on ultrasound, 14 (60.9%) cases of malignant masses were >10 cm in size, as compared to 11 (31.4%) benign cases. Overall, 65.7% of benign cases were between 5–10 cm in size, while one (2.9%) was less than 5 cm in size. One case of borderline tumor was between 5 and 10 cm in size, whereas the other was more than 10 cm in size. Significant differences were found between the groups (P<0.001) (Table 3).
In our study, after taking a detailed history and physical examination of the patient, the required investigations were performed; overall, patients were subjected to transabdominal and transvaginal ultrasound, followed by color Doppler. Contrast-enhanced computed tomography or magnetic resonance imaging was also performed in a few cases. Of the 60 selected patients, 35 (58.33%) were histopathologically confirmed to have benign masses, 23 (38.33%) patients had malignant masses, and the remaining two (3.33%) had borderline masses.
While evaluating the presenting complaints, abdominal pain (60%) was the most common presenting symptom in all three groups. Overall, 65.2% of malignant patients presented with abdominal distension and bloating, which was found to be statistically significant. With respect to benign masses, 11.4% patients indicated a history of abdominal distension and bloating, 14.3% patients had primary complaints of menstrual abnormalities, and 14.3% patients had primary complaints of infertility. It was interesting to note that 11.4% of benign cases and 4.3% of malignant cases presented with incidental findings on ultrasound, while 17.1% benign cases presented with ovarian torsion.
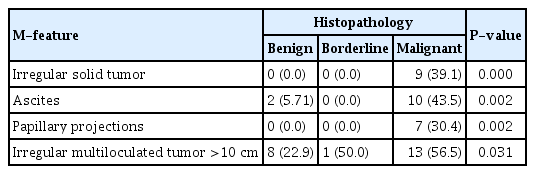
Comparison of the B-features revealed that most benign tumors presented as unilocular cysts, while none of the malignant and borderline masses showed this presentation. Further, 13 (37.1%) cases of benign tumours had solid areas of less than 7 mm, while one (4.3%) malignant tumour had solid areas of less than 7 mm. Twenty (57.1%) cases of benign tumors had acoustic shadows as compared with one (4.3%) malignant mass. Twelve (34.3%) benign cases, six (26.1%) malignant cases, and one (50.0%) borderline tumor were identified as smooth multilocular tumors less than 10 cm in size (Table 4).
With respect to M-features, nine (39.1%) malignant tumors had irregular solid areas and none of the benign masses had any irregular solid areas. Ten (43.5%) cases of malignant tumours had ascites, while two (5.71%) cases of benign tumors had ascites. Seven (30.4%) cases of malignant tumors had papillary projections. Thirteen (56.5%) malignant cases, eight (22.9%) benign cases, and one (50.0%) borderline tumor were identified as irregular multiloculated tumors greater than 10 cm in size (Table 4).
Based on the presence of M or B features, the masses were classified as benign, malignant, or inconclusive. Based on the IOTA classification, 27/35 (77.1%) benign cases, were correctly identified as benign, 6/35 (17.1%) benign cases were incorrectly identified as malignant, and two (5.7%) were found to be inconclusive. In the malignant group, 17/23 (73.9%) cases were correctly identified as malignant, three (13.0%) were wrongly judged as benign, and three (13.0%) were found to be inconclusive due to the presence of both M- and B-features. In the borderline group, one case was identified as benign, whereas the other was identified as malignant. Using the IOTA classification for diagnosing benign and malignant masses, the sensitivity, specificity, positive predictive value, and negative predictive value were 70.8%, 83.3%, 73.9%, and 81.1%, respectively (Table 5).
Comparison of the color Doppler findings revealed that 16/35 (45.7%) benign tumors had vascularity, compared to 100% in the malignant tumors. When cut off values of PI <0.8 and RI <0.6 were set, 3/35 (18.8%) benign tumors had PI of <0.8, as compared to 21 malignant tumors with PI of <0.8. Similarly, for RI, four benign tumors with vascularity had RI of <0.6 as compared to 100% of malignant tumors with RI<0.6 (Table 5, Fig. 3).
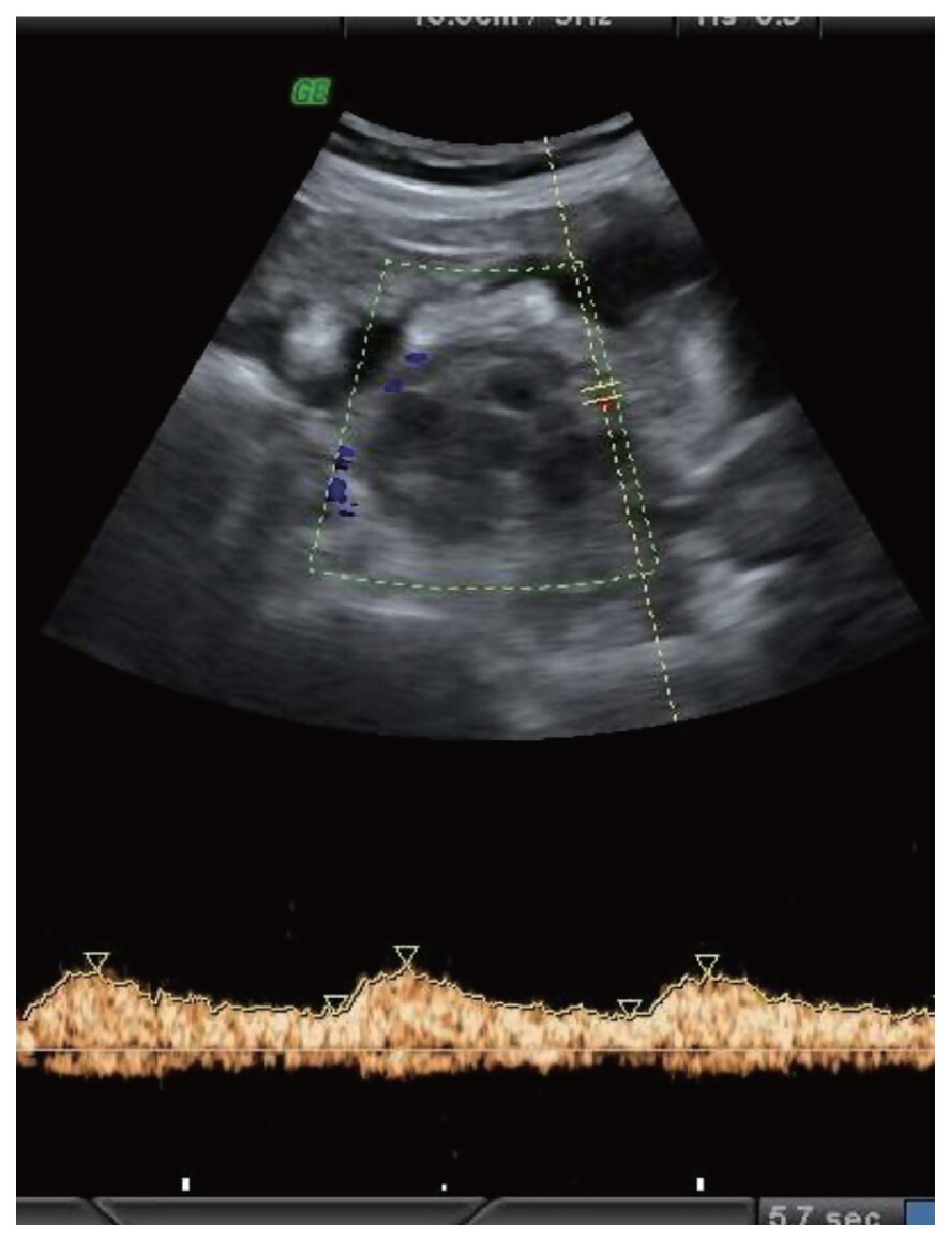
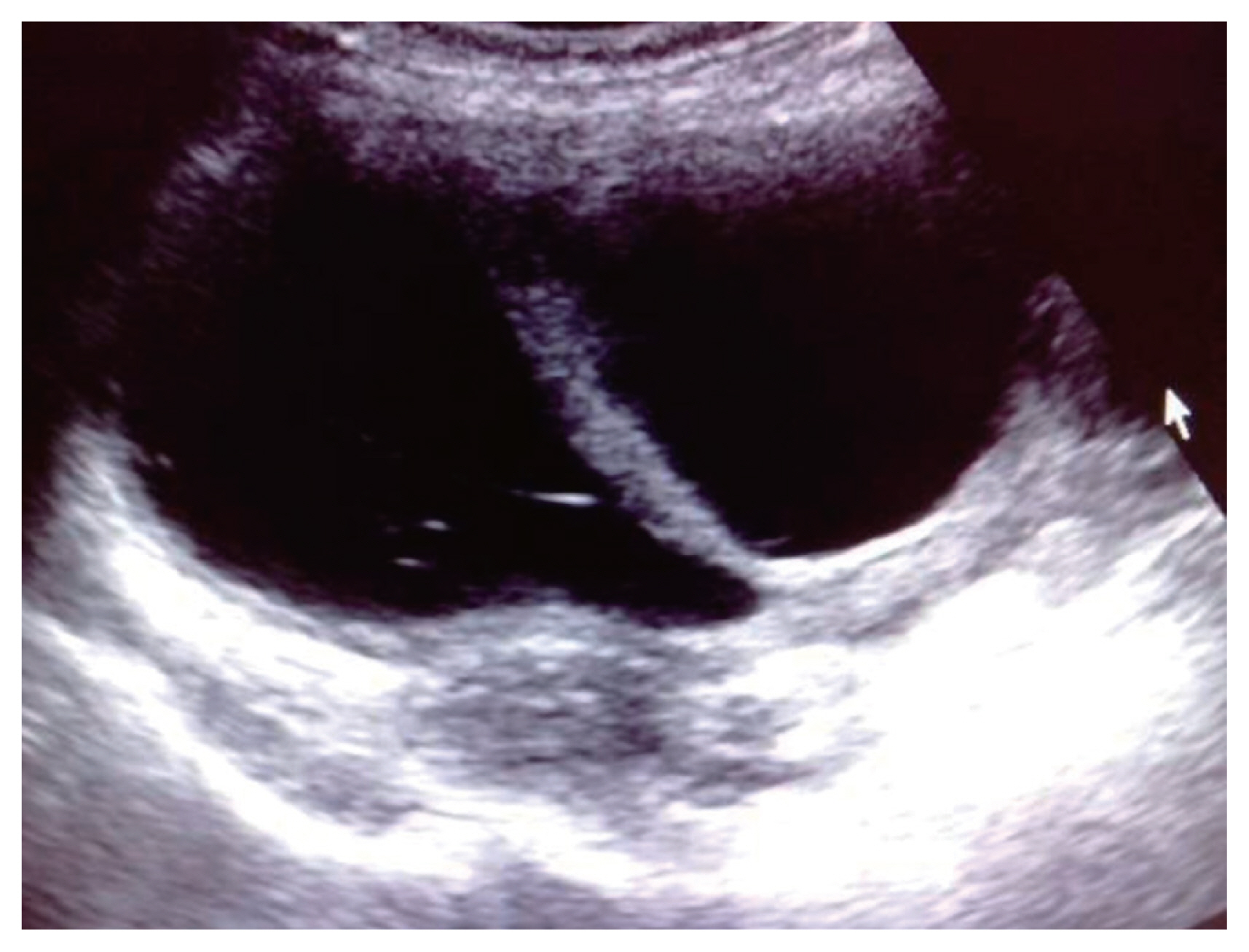
A right adnexal lesion with mixed solid and cystic areas showing low resistance vascularity consistent with Doppler features of malignancy. Ascites can be noted. Subsequent biopsy revealed mucinous cystadenocarcinoma.
Using the color Doppler method, 33/35 (94.3%) were correctly identified as benign, and 2/35 (5.7%) benign cases were incorrectly identified as malignant. In the malignant group, 19/23 (82.6%) cases were correctly identified while the rest were not. In the borderline group, one case was identified as malignant, whereas the other was identified as benign.
Discussion
Adnexal masses present a diagnostic dilemma, with many women undergoing surgery for suspicious masses, the majority of which are ultimately found to be benign. Ultrasonography is usually the first-line modality for evaluating adnexal masses [8], due to its high sensitivity for the detection and confirmation of ovarian masses, as opposed to those of uterine origin, while excluding other pathologies. The purpose of this study was to assess the diagnostic reliability of IOTA classification gray-scale ultrasound findings and color Doppler in the preoperative diagnosis of adnexal masses and to correlate them with histopathological reports. Fibroids can be a particular problem in the diagnosis of adnexal masses, as pedunculated or broad-ligament fibroid may mimic an ovarian lesion, particularly when the fibroid shows degeneration. In such instances, a full assessment, often including further magnetic resonance imaging, is appropriate [8].
The mean age of patients in this group was 46 years, and the greatest number of cases were >45 years of age. However, 55% patients who developed an adnexal mass were above the age of 45 years, oh whom 46.8% patients had benign adnexal masses and 53.1% patients had malignant adnexal masses. Hence, older age is associated with an increased incidence of ovarian cancer [9]. In a study conducted in an Indian population by Murthy from 2001–2006, a trend of increasing incidence of ovarian cancer with increasing age was observed, with age-specific rates rising from 35 years and peaking at 55–64 years [10].
The common symptoms among malignant cases in this study were bloating and fullness of the abdomen (71%) and dull aching abdominal pain or lower back pain (52%) [11]. The other common complaint was abdominal pain, while some patients were asymptomatic [11]. Regarding the ovarian involvement, 2.9% benign cases were bilateral as compared to 21.7% of malignant masses. In a study that characterized the appearance of ovarian tumors, the presence of a bilateral masses had a positive predictive value of 38% for the presence of malignancy [12].
Depending on the size of the adnexal mass on ultrasound, 60.9% of malignant masses were >10 cm in size, as compared to 31.4% of benign cases. Further, 65.7% of benign cases were between 5–10 cm in size. Earlier studies found that benign adnexal lesions had a mean diameter of 6.98 cm, while malignant adnexal lesions measured up to 9.81 cm [13]. Others have reported that benign masses were generally 5–10 cm in size and malignant masses were more than 10 cm in size [14].
On ultrasound, 77.14% of benign masses showed features corresponding to cystic lesions of less than 10 cm in size and 22.9% benign tumors were cystic lesions of more than 10 cm in size. A further 45.7% of benign cases had thin septations and 42.8% had no septations. Conversely, malignant masses were mostly solid in consistency; 39.1% of malignant tumors were irregular solid tumors and 65.5% of malignant tumors were multiloculated tumors of more than 10 cm in size with large solid areas. A further 52.17% of malignant tumors had thick septations. These findings agree with the results of a prior study which showed that on ultrasound, the majority of benign masses were cystic in consistency (90%), with thin septations of less than 3 mm (53.57%), while most malignant masses were predominantly solid (76%), with thick septations of more than 3 mm (63.63%) [15].
In our study, 30.4% of malignant tumors had papillary projections. 83.33% of patients with ascites had malignancy. 43.5% cases of malignant tumors had ascites, whereas 5.71% benign tumours of mucinous cystadenomas had ascites. Prior studies have similarly found that papillary projections and ascites are indicative of malignancy [16]. Others have also noted that 41% cases of stage IV epithelial ovarian cancer have papillary projections [17].
In the present study, the IOTA classification was found to have a sensitivity, specificity, positive predictive value, and negative predictive value were 70.8%, 83.3%, 73.9%, and 81.1%, respectively, in the diagnosis of benign and malignant masses. In a prior study using the IOTA classification, the sensitivity, specificity, positive predictive value, and negative predictive value were found to be 91.66%, 84.84%, 68.75%, and 96.55%, respectively [18].
Comparison of the color Doppler findings revealed that 45.7% of the benign tumors had vascularity, while the rest did not. In our study, color Doppler showed 100% vascularity in malignant tumours and borderline tumours. Prior studies have demonstrated that the absence of blood flow is suggestive of malignancy [19]. Another study showed that color Doppler evaluation of the tumor revealed the presence of vascularization in 97.5% of malignant tumors, as opposed to 68.1% benign tumors [20].
When the value of RI <0.6 was taken as an indicator of malignancy; 11.4% benign tumours had RI of <0.6, and 100% of malignant tumors had RI <0.6. In a previous study, an RI of <0.6 was seen in 6.81% benign tumors and 82.5% of malignant tumors [20]. In our study, a cut off value of RI <0.6, had a sensitivity, specificity, positive predictive value, and negative predictive value of 82.1%, 100%, 100%, and 72.2%, respectively. In another study the results showed that using a cut off resistance index value of 0.6, the sensitivity and specificity of color Doppler in the detection of malignant tumors were 82% and 72%, respectively [21].
A cut off value of PI <0.8 has been considered as an indicator of malignant lesions as per a study 8.5% of benign tumors had PI of <0.8, compared to 91.3% of malignant tumors with PI of <0.8 [22]. In our study using a cut-off PI <0.8, the sensitivity of PI was 84%, specificity of 87.5%, positive predictive value 91.3%, and negative predictive value of 77.8%.
A previous study performed by Mallari and coloma [22] showed no significant difference in distinguishing benign from malignant neoplasms sonographically between Sassone scoring and ADNEX model, with accuracy rates of 88% and 89% respectively. Similarly, Timor-Tritsch et al. [19] created a scoring system by excluding the wall thickness parameter from the Sassone scoring system while adding shadowing as another scoring system, to discriminate dermoid cysts from malignant adnexal masses. The Lerner scoring system using a cut off score of 3 was reported to have a high sensitivity of 96.8% and a lower specificity of 77% [23].
Preoperative evaluation of the expected extent of the surgical approach is paramount in the selection of surgical candidates. Elderly patients with gross ascites and upper abdominal involvement with abdominal and pleural metastases should be evaluated using a multidisciplinary team approach [24]. The primary limitation of this study is that we were only able to include a small number of patients based on the exclusion criteria.
This study showed that age was a significant risk factor for ovarian malignancy. Postmenopausal status is consistently associated with an increased risk of malignancy. Overall, a CA-125 of ≥35 IU/mL was found to be indicative of malignant potential. The IOTA classification is a reliable morphological scoring system that can be used as a primary diagnostic modality for adnexal masses and can minimize interobserver variation [25]. In color Doppler sonography, the presence of vascularity (RI <0.6 and PI <0.8) was found to correlate with malignancy. Vascularity was the most sensitive parameter, while PI and RI were equally sensitive, although PI was more specific for the detection of malignancy. The IOTA classification system significantly improved the preoperative diagnosis of benign versus malignant ovarian neoplasms and correlated more accurately with histopathology.
Notes
Conflict of interest
Nil.
Ethical approval
IEC approval was done (IEC KMC MLR 08-17/144).
Patient consent
Is available.
Funding information
No funding for study.