Primary ovarian choriocarcinoma mimicking ectopic pregnancy
Article information
Abstract
Nongestational ovarian choriocarcinoma is an exceedingly rare and highly aggressive tumor. Although early diagnosis and timely initiation of therapy is important, it is difficult in reproductive aged patients because of the frequent elevation of human chorionic gonadotropin. We report a primarily nongestational ovarian choriocarcinoma in a 12-year-old virgin female. Initial diagnosis based on abdominopelvic computed tomography and pelvis magnetic resonance imaging was ectopic pregnancy with hemoperitoneum. A diagnostic laparoscopy of the ovarian tumor revealed choriocarcinoma. Unilateral salpingo-oophorectomy and omental sampling revealed surgical stage of IA. Six courses of adjuvant combination chemotherapy (bleomycin, etoposide, and cisplatin) followed surgery.
Introduction
Ovarian choriocarcinoma is a rare and highly aggressive tumor. It may develop as a metastatic gestational choriocarcinoma from uterus or tubal choriocarcinoma, as a primary gestational choriocarcinoma associated with ovarian pregnancy, or as a nongestational germ cell tumor differentiating towards the trophoblastic structures [1]. The gestational type is more common than the nongestational type; the estimated incidence of a primary ovarian choriocarcinoma is 1 in 389,000,000. They comprise 2.1% of all malignant ovarian germ cell neoplasms [2]. To our knowledge, 6 cases of nongestational ovarian choriocarcinoma have been reported in Korea.
Gestational choriocarcinoma is associated with the gestational trophoblastic disease spectrum. Nongestational ovarian choriocarcinoma is associated with the transformation of a single germ cell. Both types of choriocarcinoma tend to develop early hematogenous metastasis to several different sites that include the lung, liver, brain, bone, vagina, and other viscera. Compared to gestational choriocarcinomas, nongestational choriocarcinomas are relatively chemoresistant and are associated with an unfavorable prognosis, especially in the advanced stage [1]. Therefore, early diagnosis and timely initiation of therapy is important. However, early diagnosis presents unique challenges in reproductive-age women.
This report describes a case that mimicked an ectopic pregnancy, which was finally diagnosed as nongestational ovarian choriocarcinoma. The patient was managed with laparoscopic ipsilateral salpingo-oophorectomy and surgical staging, followed by the administration of combination chemotherapy.
Case report
A 12-year-old virgin woman presented with a 20-day history of vaginal bleeding and one-day duration of lower left quadrant abdominal pain. Her last menstrual period was 1 month ago and perimenstrual period was irregular. She had a pelvic ultrasonography, abdominopelvic computed tomography and pelvis magnetic resonance imaging, and was tested for urine human chorionic gonadotropin (hCG) at Samsung Changwon Hospital. She was transferred to our hospital under the impression of ovarian malignant tumor.
Examination at our hospital revealed increased serum hCG level (20,257 mIU/mL) and increased lactate dehydrogenase level (421 mg/dL). Other tumor markers including CA-125, CA-19-9, and alpha-fetoprotein were within normal ranges. Pelvic ultrasonography revealed a 3.83×3.43 cm sized solid, cystic, heterogeneous lesion on the left ovary. Abdominopelvic computed tomography and pelvis magnetic resonance imaging revealed an approximately 2.8 cm sized hemorrhagic cystic lesion with enhancing portion and enhancing salpinx, left ovary and suspected ectopic pregnancy (Fig. 1A).
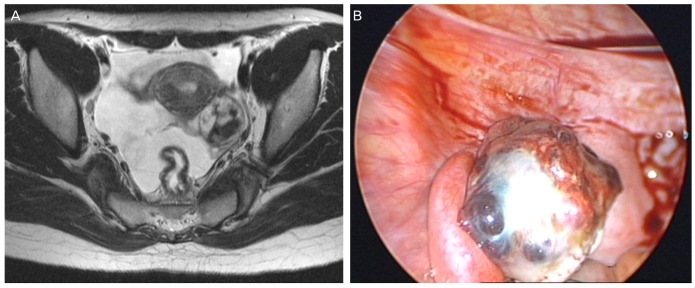
(A) Pelvis magnetic resonance imaging T2 image shows an approximately 2.8 cm sized hemorrhagic cystic lesion with enhancing portion and enhancing salpinx in the left ovary. (B) Laparoscopic views show a cystic and solid mass about 5×3 cm in size.
A diagnostic laparoscopy was performed on June 12, 2012. Intraoperative assessment revealed a normal appearing uterus and right adnexal structures, and a hen's egg-sized solid and cystic mass with an irregular surface in the left ovary and mild hemoperitoneum (Fig. 1B). Inspection of all other viscera and peritoneal surfaces failed to identify any suspicious lesion. Frozen section examination of the tumor revealed a malignant germ cell tumor. The patient underwent laparoscopic left salpingo-oophorectomy, partial omentectomy, and multiple peritoneal biopsies.
Detailed pathologic examination revealed a mixed germ cell tumor consisting of choriocarcinoma (90%) and dysgerminoma (10%) (Fig. 2). Biopsy of the omentum and peritoneum showed no evidence of metastasis. Surgical pathological stage was IA. Six courses of adjuvant chemotherapy consisting of bleomycin, etoposide, and cisplatin (BEP) were completed in November 2012 without major complications. The hCG level was normalized within 35 days postoperatively and the patient has been without clinical or biochemical evidence of disease for 14 months.

(A) Gross appearance of the ovarian tumor. The cut surface shows papillary and solid portion with focal hemorrhage. (B) Microscopic finding of the choriocarcinoma shows a biphasic arrangement of cytotrophoblasts and syncytiotrophoblasts (H&E stain, ×200). (C) The choriocarcinoma component shows diffuse strong reactivity with beta-human chorionic gonadotropin by immunohistochemistry (×100). (D) The dysgerminoma component shows reactivity with octamer transcription factor 3/4 by immunohistochemistry (×200).
Discussion
Nongestational choriocarcinoma is an exceedingly rare malignant tumor of the ovary. While occasionally detected as a homogeneous lesion, other germ cell components are frequently present. Unfortunately, this biologically aggressive tumor is commonly found in young woman less than 20 years of age. In a review of the literature on primary choriocarcinoma of ovary, the age range of the patients was 7 months to 35 years with a mean of about 13 years [3].
Early-stage ovarian choriocarcinoma presents a significant diagnostic challenge in the reproductive-aged patient because of elevated hCG. While the differential diagnosis includes both an ectopic pregnancy and gestational trophoblastic disease, nongestational choriocarcinoma is not often a consideration. Abnormal vaginal bleeding, abdominal pain, and detection of pelvic mass may be common but with nonspecific complaints and signs that only reflect high hCG levels. Similar to gestational choriocarcinomas, lung or brain metastases has been described [4]. Isosexual precocity has been reported to occur in about 50% of patients whose lesions appear before menarche. hCG maybe the most sensitive measurement in diagnosing and monitoring the response to treatment.
The two types of tumor are difficult to differentiate by routine histological examination, especially in the pure ovarian choriocarcinoma without other components of germ cell tumors. DNA polymorphism analysis is a useful method to distinguish these two subtypes [1].
Diagnostic laparoscopy is invaluable in the differential diagnosis between early stages of primary ovarian choriocarcinoma and other diseases with elevated hCG in reproductive-aged women. If the tumor is confined to the ovary, staging procedures (which include infracolic omentectomy, biopsy of the diaphragmatic peritoneum, paracolic gutters, pelvic peritoneum, and peritoneal washings) can be done laparoscopically. There is no consensus about the role of systematic lymphadenectomy [5]. In recent study, Colombo et al. [6] suggested that node dissection consider only in those cases with suspected nodal metastasis. Systematic ovarian biopsy avoid when the contralateral ovary is macroscopically normal.
As nongestational choriocarcinoma usually occurs in childhood and adolescence, fertility saving surgery including comprehensive surgical staging consider in treating young patients. Even in advanced disease, fertility saving surgery can be considered, because of the sensitivity of the tumor to chemotherapy. In postmenopausal women and in patients with advanced stage disease or with bilateral ovarian involvement, abdominal hysterectomy and bilateral salpingo-oophorectomy consider performed with careful surgical staging [6,7].
Since nongestational choriocarcinoma is considered as a germ cell tumor differentiating to trophoblastic components, a germ cell tumor treatment protocol may be effective. Since 1989, a regimen involving BEP has become standard for all ovarian germ cell malignancies [8]. In addition to higher cure rates, this chemotherapy regimen preserves normal menstrual function and can maintain fertility with healthy offspring in malignant ovarian germ cell tumor cases [9].
In conclusion, primary ovarian choriocarcinoma should be included in the differential diagnosis of ovarian lesions in childhood and adolescence, despite its extreme rarity. The early stage of an ovarian choriocarcinoma can be treated effectively with fertility saving surgery combined with multiple courses of BEP chemotherapy.
Notes
No potential conflict of interest relevant to this article was reported.