Atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesion triage in Korean women: Revisiting the 2012 American Society of Colposcopy and Cervical Pathology screening guidelines
Article information
Abstract
Objective
To determine whether triage for atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesion (LSIL) from the updated American Society for Colposcopy and Cervical Pathology cervical cancer screening guidelines is applicable in Korean women.
Methods
We investigated women with ASC-US or LSIL including referred from local hospitals visited for cervical cancer screening at Korea University Guro Hospital from February 2004 to December 2014. Detailed information on the results of Papanicolaou (Pap) smears, human papillomavirus (HPV) DNA tests, and cervical biopsies were collected through chart review. Cervical biopsy results were compared in eligible women according to individual Pap smear findings and HPV DNA status.
Results
Of 216,723 possible cases, 3,196 were included. There were 212 (6.6%) women with ASC-US and 500 (15.6%) with LSIL. The risk of ≥cervical intraepithelial neoplasia (CIN) 2 was significantly higher in women who were ASC-US/HPV+ than ASC-US/HPV- and LSIL/HPV+ than LSIL/HPV- (93.3% vs. 6.7% and 96.7% vs. 3.3%, P<0.001 and P<0.001, respectively). The risk of ≥CIN 3 was also significantly higher in women who were ASC-US/HPV+ than ASC-US/HPV- and LSIL/HPV+ than LSIL/HPV- (97.0% vs. 3.0% and 93.0% vs. 7.0%, P<0.001 and P<0.001, respectively). Age-stratified analysis revealed that more CIN 2 or CIN 3 was diagnosed in women aged 30 to 70 with ASC-US or LSIL when HPV DNA was present.
Conclusion
Observation with Pap and HPV DNA tests rather than immediate colposcopy is a reasonable strategy for ASC-US or LSIL when the HPV DNA test is negative, especially in women aged 30 to 70. Reflection of these results should be considered in future Korean screening guidelines.
Introduction
Although the incidence of cervical cancer in Korea has recently declined, it still ranks higher than in more developed countries [12]. Government data estimate that it was the seventh most common female cancer in 2015, with 3,100 new cases, and the third most common cancer in women aged 15 to 34 years [345]. Preinvasive or carcinoma in situ is on the rise compared to invasive carcinoma in Korea, especially in young women [6].
In 2013, the Korean Society of Gynecologic Oncology (KSGO) issued practice guidelines for the early detection of cervical cancer [7]. Various kinds of guidelines were reviewed in the process of publication, and most included guidelines developed by the American Society for Colposcopy and Cervical Pathology (ASCCP) (ver. 2006) [8910]. The ASCCP guidelines were renewed right after publication of the KSGO guidelines [11]. Differences in the management of cytological atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSILs) stand out as being more important than the rest. Namely, the revised ASCCP guidelines avoid ‘immediate colposcopy’ in ASC-US, and recommend repeating cytology in human papillomavirus (HPV)-negative patients rather than colposcopy in LSIL.
There is some hesitance to accept the updated ASCCP guidelines because of the high prevalence rate of cervical cancer and low medical costs in Korea [7]. However, cytological ASC-US and LSIL are somewhat heterogeneous entities in terms of correlation with high-grade pathology [12]. Therefore, we evaluated the correlation between ASC-US and LSIL cytology and pathological results according to HPV status in Korean women to confirm the applicability of the updated ASCCP guidelines in Korea.
Materials and methods
We investigated women with ASC-US or LSIL including referred from local hospitals visited for cervical cancer screening at Korea University Guro Hospital from February 2004 to December 2014. Results of Papanicolaou (Pap) smears, HPV DNA tests, and cervical biopsies were extracted by chart review. Cases with duplicated data or lacking biopsy or HPV DNA test results were excluded. The Korea University Guro Hospital institutional review board approved this study (KUGH16056). The need for obtaining informed consent was waived.
For Pap tests, liquid-based cytology (ThinPrep, Cytyc Corp., Marlborough, MA, USA) was used. Cervical samples were collected using a Cervex-Brush (Rovers Medical Devices, Oss, The Netherlands) from the cervical os. For HPV detection, the Hybrid Capture 2 test (Digene Corp., Gaithersburg, MD, USA), which can detect 13 high-risk HPVs, was used [13].
In case of abnormal Pap smear results or HPV DNA positivity, a colposcopy-directed biopsy was performed. Colposcopy-directed biopsies were performed by skilled gynecologic oncologists with more than 10 years of specialized experience in our institution. Categorical variables are indicated as numbers and percentages. To compare the risk of having high-grade cervical intraepithelial neoplasia (CIN) in women with ASC-US or LSIL according to HPV status, Pearson's chi-square test was used. Statistical analyses were performed with SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). All P-values <0.05 were considered statistically significant.
Results
A total of 43,964 women were enrolled (Fig. 1). The number of women with cytological ASC-US and LSIL was 27,537 and 16,427, respectively. Following exclusion of women who lacked either HPV or biopsy results or were counted twice, 712 women were included in the study.
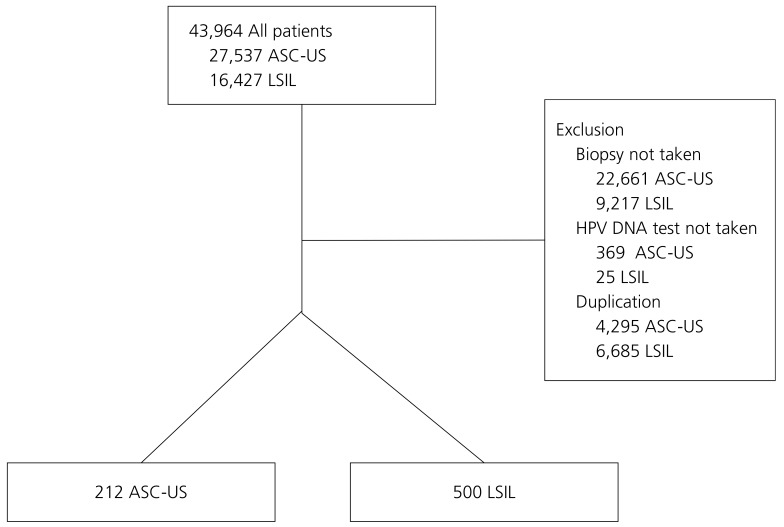
Flow diagram showing patient enrollment. Exclusion criteria and final enrollment status are demonstrated. ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HPV, human papilloma virus.
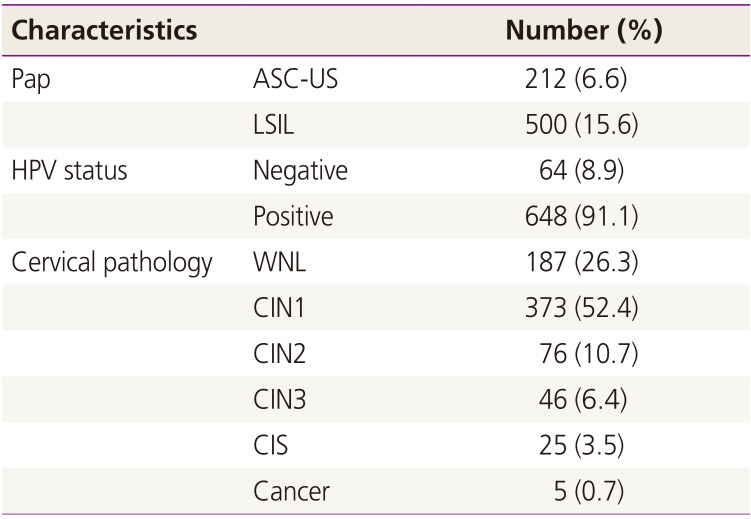
Baseline characteristics of the study population are described in Table 1. The median age was 47 years (range, 15 to 86 years). As expected, over two thirds of women were cytologically normal. The number of women with ASC-US and LSIL was 212 (6.6%) and 500 (15.6%), respectively. The median age of women with ASC-US and LSIL was 48 years (range, 20 to 82 years) and 39 years (range, 15 to 79 years), respectively. On the HPV test, 648 (91.1%) women were HPV positive. According to colposcopy-directed biopsy results, 147 (20.6%) women were confirmed to have high-grade CIN. Pathologically-confirmed invasive cancer was found in only 5 (0.7%) women.
As cytological results got worse from ASC-US to HSIL, cervical pathology results corresponded. In women with ≤CIN 1, more had ≤LSIL than HSIL (578 [93.2%] vs. 42 [6.8%], respectively, P=0.001). In contrast, in women with ≥CIN 2, the number with HSIL was higher than those with ≤LSIL (264 [61.3%] vs. 167 [38.7%], respectively, P=0.001). The results were similar when women were divided into ≤CIN 2 and ≥CIN 3. In women with ≤CIN 2, more had ≤LSIL than HSIL (656 [93.9%] vs. 42 [6.1%], respectively, P=0.001). In contrast, in women with ≥CIN 3, the number with HSIL was higher than those with ≤LSIL (202 [69.4%] vs. 89 [30.6%], respectively, P=0.001). The proportion of high-grade CIN in women with ASC-US or LSIL according to HPV status is shown in Table 2. In women with ASC-US, significantly more ≥CIN 2 was identified when HPV test results were positive than when they were negative (93.3% vs. 6.7%, P<0.001). These same results could also be seen in cases of ≥CIN 3 (97.0% for HPV-positive vs. 3.0% for HPV-negative, P<0.001). In women with LSIL, significantly more ≥CIN 2 and ≥CIN 3 could be found when HPV test results were positive than when they were negative (96.7% vs. 3.3%, 93.0% vs. 7.0%, P<0.001 and P<0.001, respectively).
According to the KSGO screening guidelines, HPV DNA tests are recommended for women between the ages of 30 and 70. For these reasons, subgroup analysis was done in women aged 30 to 70 to evaluate the effect of HPV status on cervical pathology in cytological ASC-US and LSIL (Table 2). High-grade CINs were found significantly more frequently when HPV was positive than negative in cytological ASC-US and LSIL (P<0.001). Of note, no ≥CIN 3 was identified when HPV was negative in women with cytological ASC-US.
Discussion
No screening test can prevent all cancers by itself. A combination of tests that are complementary to one another offers a more realistic approach [14]. Because the false-negative rate of Pap smears is quite high, cytology alone is not sufficient to detect women who might have precancerous lesions [15]. Epidemiologic case series have shown that nearly 100% of cervical cancer cases test positive for HPV [16]. Thus, HPV and Pap co-testing can be used to raise the quality of clinical prediction.
Our findings focused on whether Pap test results actually revealed abnormal pathology based on HPV DNA test results. Cytological ASC-US or LSIL presented significantly higher-grade CIN in HPV positive cases than HPV negative cases. In other words, women with ASC-US or LSIL without high-risk HPV infection seldom harbor high-grade CIN. According to KSGO guidelines for cervical cancer screening, there are three management options following ASC-US on Pap smear: (1) repeat Pap test, (2) HPV DNA test, or (3) immediate colposcopy. In cases of LSIL, colposcopy is the only choice [8]. On the other hand, the revised 2012 ASCCP no longer recommend immediate colposcopy for cytological ASC-US [11]. HPV testing is preferred, but repeat cytology in one year is also acceptable. Moreover, for LSIL, repeat cytology is preferred when HPV is negative, although immediate colposcopy is still acceptable. Our findings in this study strongly support these recommendations. Although it provides high-quality screening, colposcopy is a somewhat invasive and costly technique that requires skilled technicians and specific equipment [17]. As there is less harsh situation in Korea than that of United States, we still prefer to have an immediate colposcopy rather than wait for continuous negative test findings. However, there is a lack of evidence that immediate colposcopy is superior to observation and repeat tests, and our findings support a more patient approach [18].
Our study has several limitations. Our findings may be difficult to generalize to the Korean population as a whole because our study was conducted at a single university hospital. Errors from selection bias were also unavoidable, since the conclusion was somewhat expected. In addition, we excluded more women than expected due to a lack of biopsy results, which may have been attributed to follow-up loss, patient refusal, or performance of repeat cytology that was confirmed as normal thereafter.
Despite these limitations, our study was relatively large-scale, with more than 3,000 patients, from a single hospital unit in an inner city. There have been no other studies focused on differences between revised ASCCP guidelines and existing guidelines in Korea for cervical cancer.
In conclusion, screening test guidelines from different countries should be weighed to see if they are applicable to the respective countries. This study was intended to examine whether HPV test results could inform clinical decisions in cytological ASC-US or LSIL. We found that ASC-US or LSIL findings without evidence of positive HPV do not require immediate colposcopy. Observation with Pap and HPV DNA tests is a reasonable strategy for ASC-US or LSIL when the HPV DNA test is negative. Existing screening guidelines in Korea should be amended to reflect these results.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.