Leiomyoma development in Mayer-Rokitansky-Küster-Hauser syndrome: a case report and a narrative review of the literature
Article information
Abstract
The development of leiomyomas on the grounds of an aplastic/hypoplastic uterus in patients with Mayer-Rokitansky-Küster-Hauser syndrome (MRKHS) has been rarely described. We report the first case of development of multiple leiomyomas in a patient with MRKHS complicated with pulmonary valve stenosis, and we present a narrative review of the existing literature. A 44-year-old patient with MRKHS attended our clinic because of pelvic pain, which was attributed to a pelvic mass found on ultrasound. Magnetic resonance imaging revealed a multinodular mass, indicating either ovarian pathology or the presence of leiomyomas. Exploratory laparotomy was performed, and multiple solid masses on the grounds of two rudimentary uterine buds were observed. Histological analysis revealed multiple leiomyomas arising from parametrial or paratubal tissue. We searched medical databases for articles relevant to leiomyomas and MRKHS. We present a review of the current literature and summarize the clinical manifestation, diagnosis, management, and histopathological findings of all the cases described. We underline that it is important for gynecologists to be aware of this rare clinical entity, and symptomatic leiomyomas cannot be excluded in patients with MRKHS.
Introduction
Mayer-Rokitansky-Küster-Hauser syndrome (MRKHS) is a congenital disorder characterized by uterovaginal aplasia/hypoplasia due to the unsuccessful fusion of the two Müllerian ducts early in the embryological development. The syndrome occurs in 1 in 4,000–5,000 live female births and it is the second most common cause of primary amenorrhea. Specific congenital abnormalities of the urinary, skeletal, auditory, and cardiac systems have been secondarily associated with some subtypes of the syndrome [1].
In the current literature, 35 patients with MRKHS with development of leiomyoma are described. Here, we report a rare case of development of multiple leiomyomas in a patient with MRKHS complicated with skeletal and cardiac anomalies. In addition, we present a narrative review of the literature concerning the development of leiomyomas in patients with MRKHS.
Case report
A 44-year-old woman with MRKHS attended our outpatient clinic for further investigation and treatment of a pelvic mass. The patient had been diagnosed as having MRKHS at the age of 14 years, when she first sought medical advice for primary amenorrhea. She had a surgical history of open pulmonary valvotomy under cardiopulmonary bypass due to pulmonary valvar stenosis (at the age of 11), and creation of a neovagina in accordance with the Creatsas modification of Williams vaginoplasty (at the age of 16) [2]. As for the skeletal system, mild kyphosis had been diagnosed at the age of 15.
In 2016, she was admitted to our clinic because of a pelvic mass arising potentially from the ovaries as indicated by a pelvic ultrasound, accompanied with a constant, pelvic pain lasting for 3 months. No urinary or bowel complaints were mentioned. On physical examination, secondary sexual characteristics as well as the external genitalia were found to be well developed. Bimanual examination revealed a solid, rough, mobile, suprapubic mass of approximately 10 cm in diameter. Tumor markers were all within normal limits, and hormonal profile results indicated a normal ovarian function.
Pelvic magnetic resonance imaging (MRI) findings were consistent with a multinodular mass measuring 92×79 mm that was comprised of smaller, discrete masses. It was impossible to differentiate whether the mass arose from the ovaries or a hypoplastic uterus.
Exploratory laparotomy revealed two rudimentary uterine buds joined together in a midline fibrous band with multiple solid masses on it, grossly resembling fibroids (Fig. 1). Ovaries and fallopian tubes looked normal, whereas the cervix could not be palpated. Excision of the redundant piece of uterine tissue, including the tumors, as well as bilateral salpingo-oophorectomy was performed. After an uneventful recovery, the patient was discharged 6 days after surgery.
The histopathological report revealed normal ovaries and fallopian tubes, complete aplasia of the uterus, and multiple leiomyomas arising from either parametrial or paratubal tissue.
Discussion
MRKHS results from a failure of the development of the Müllerian duct system. Although the majority of cases are sporadic, familiar cases indicate a potential autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. No specific causative gene is identified [3]. Historically, the consolidation of this specific and heterogenous clinical entity started in 1829 by August-Frauz-Joseph-Kark-Mayer and was completed in 1961 by George-Ardie-Hauser.
MRKHS is characterized by the absence of the upper two-thirds of the vagina, a hypoplastic/aplastic uterus, and well-developed ovaries and fallopian tubes in patients with a normal 46XX karyotype and normal secondary sexual characteristics. MRKHS is classified as typical-type in case of an isolated, symmetrical uterovaginal aplasia/hypoplasia, or atypical-type 2 when additional congenital malformations are present. Common congenital disorders include skeletal, renal, and heart malformations as well as hearing impairment, but more unusual disorders have also been reported [4].
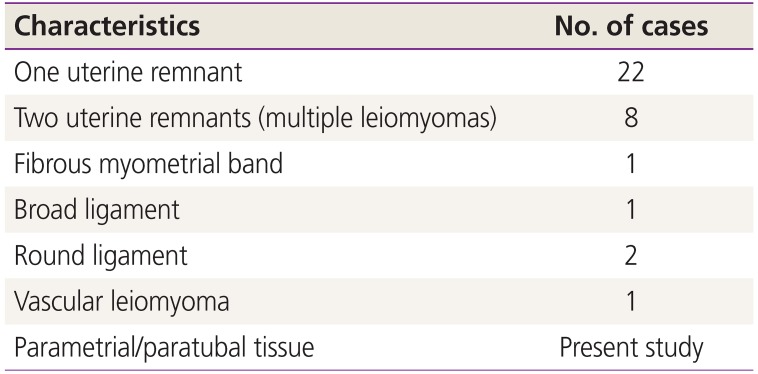
Regardless of partial or complete aplasia of the uterus, leiomyomas have been described in 35 patients with MRKHS. The first case of myoma in association with MRKHS was described by Beecham and Skiendzielewski [5] in 1977. Concerning the origin of the myomas, the majority arose from the fibromuscular tissue of one uterine remnant (22 cases). Vascular leiomyomas, myomas arising from the broad or the round ligament, as well as multiple myomas originating from both rudimentary ducts or a fibrous myometrial band have also been described (Table 1). In our case, complete aplasia of the uterus was confirmed, and multiple leiomyomas had developed from parametrial or paratubal tissue, which has never before been reported in the literature.
Concerning the pathogenesis, like in a normal uterus, the fibromuscular tissue is a target of steroids produced by the functional ovaries. The development of multiple fibroids in a postmenopausal woman with MRKHS who was taking hormone replacement therapy indicated the role of hormonal stimulation in the development of fibroids [6].
Based on a literature review, we found that the median age of MRKHS patients is 39.4 years (range, 16–70 years). From all reported cases, only 3 patients had secondary congenital abnormalities of the skeletal or urinary system (kidney agenesis, external urinary meatus, prolapsed lumbar disc) [78]. We present the first case of leiomyoma development complicated with pulmonary vulvar stenosis in a patient with MRKHS. This congenital heart disorder has been reported only once in a MRKHS patient [9].
The initial diagnosis of the pelvic mass is generally made either during a regular gynecologic follow-up of an asymptomatic patient or because of acute/chronic symptomatology. Specifically, chronic pelvic pain, increasing abdominal swelling, change of bowel habits or the frequency of the micturition are some common presentations. Acute pain has been described in 5 patients with acute torsion of the mass and the respective uterine remnant, combined or not with a torsion of the adjacent ovary [8]. Chronic torsion of the ovary has been described in one case [10]. Ovarian and uterine pathology can also be found in these patients. More specifically, a Brenner tumor and a mucinous cystadenoma of the ovary, as well as adenomyosis, have been described in combination with myomas in patients with MRKHS. A clinician should include the possibility of either an ovarian malignancy [11] or a uterine sarcoma [12] in the differential diagnosis. Two cases of mitotically active leiomyomas in MRKHS without cellular atypia and a low metastatic rate to be regarded as sarcoma are described in the current literature [1314].
Differential diagnosis of a pelvic mass in a patient with MRKHS includes leiomyoma, ovarian tumors, or a mass arising from the bowel or the bladder. Bimanual examination, pelvic ultrasound, and pelvic MRI may be helpful to differentiate a myoma. Moreover, three-dimensional computed tomography has been used to evaluate the blood supply of the myoma and to subsequently determine its origin and anatomical characteristics [10]. Diagnostic laparoscopy can be used if the scans are not diagnostic.
In the majority of cases reported in the current literature, an exploratory laparotomy was performed. Excision of the myoma and the adjacent uterine remnant is the minimum surgical intervention. It is indicated to remove both uterine remnants so as to prevent recurrence [15], with or without salpingo-oophorectomy. Concering minimal invasive approach, 4 cases have been managed laparoscopically, the first of which was described in 2000. Despite the well-established benefits of minimally invasive techniques, no robotic surgery has yet been performed.
Although the development of leiomyomas in patients with MRKHS is rare, every gynecologist should be aware of this pathology. We described such a rare case and its management, and provided a narrative review of the current literature on MRKHS.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Ethical approval: The study was approved by the Institutional Review Board of Metaxa Memorial Cancer Hospital (No. 13202) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent: The patients provided written informed consent for the publication and the use of their images.