Association between different dual trigger dosages and in vitro fertilization results in patients with patient-oriented strategies encompassing individualized oocyte number group IV
Article information
Abstract
Objective
Dual trigger is used to induce final oocyte maturation during the process of controlled ovarian hyperstimulation, yet yielding controversial results. Also, there are yet no data regarding the effect of the dosage of the dual trigger on clinical outcomes. Based on the Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) criteria, this study aimed to determine the clinical difference of a single bolus versus two boluses of gonadotropin-releasing hormone agonist (GnRHa) in POSEIDON group IV patients using dual trigger.
Methods
We screened a total of 1,256 patients who underwent in vitro fertilization (IVF) cycles who met the POSEIDON group IV criteria. Six hundred and twenty-nine patients received one bolus of GnRHa, and 627 patients were given two boluses. All patients received the same dose of recombinant human chorionic gonadotropin during the dual trigger cycle.
Results
Metaphase II oocyte retrieval rate, fertilization rate and clinical pregnancy rate did not differ between the two groups. However, a lower percentage of at least one top-quality embryo transfer (34.3% vs. 26.0%, P=0.001) in the two bolus-GnRHa group was noted.
Conclusion
A double bolus of GnRHa did not show superior clinical results compared to a single bolus of GnRHa in the dual trigger IVF cycle. Therefore, GnRHa doses for use should be decided based on individual clinical situations considering cost-effectiveness and patient compliance, but further investigation will be needed.
Introduction
Human chorionic gonadotropin (hCG) has been traditionally favored by clinicians as a substitute for luteinizing hormone (LH) in the process of in vitro fertilization (IVF) cycles due to its similar biological and molecular nature with LH [1]. hCG is administered to mimic a natural LH surge, which is absent in IVF cycles, stimulated by gonadotropin-releasing hormone (GnRH) agonists in controlled ovarian hyperstimulation (COH) [2]. The final oocyte maturation occurs in the mid-ovulatory cycle after the LH surge, which involves the progression of metaphase II (MII), the resumption and completion of meiotic division, and luteinizing of the granulosa cells [3].
GnRH agonists (GnRHa) for the final oocyte maturation were first suggested for their capability to trigger both follicle-stimulating hormone (FSH) and LH surge and shorter durations (12–36 hours) compared to hCG trigger [4,5]. However, previous studies showed that isolated GnRHa triggers resulted in complications such as lower live birth rate, higher early miscarriage rate, lower ongoing pregnancy rate, and an increased risk of empty follicle syndrome [6,7]. In this context, co-administration of GnRHa and hCG for the final oocyte maturation 36 hours before oocyte retrieval was introduced, known today as “dual trigger” [8].
To date, numerous studies have reported the positive effects of dual trigger, one of which showed a higher live birth rate in normal responders [9]. In another study, increases in the numbers of retrieved oocytes, MII oocytes, embryo implantation, and clinical pregnancy rates were noted [10]. Also, a retrospective study revealed that the percentage of mature oocytes and number of top-quality embryos were either comparable or higher in the GnRHa group [11].
For patients with poor ovarian response (POR), evidence showed that IVF results were worse than normal responders, resulting from a reduced number of follicles [12]. The Bologna criteria were established in 2011 as the international standard of POR [13], but due to its high heterogeneity, the clinical applicability is still in question. Therefore, the Patient-oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) criteria were developed in 2016 [14]. This new system puts emphasis on a better stratification of patients with low prognosis, and more individualized therapeutic schemes for each group [15]. Four subgroups based on quantitative and qualitative parameters have been suggested; 1) age and the expected aneuploidy rate, which represents oocyte quality, 2) ovarian biomarkers (antral follicle count [AFC] and anti-Müllerian hormone [AMH]), which represents oocyte quantity, and 3) ovarian response, meaning the number of retrieved oocytes in the previous stimulation cycle. The aforementioned classification provides more precise clinical recommendations for the treating physicians. Our target patient group is targeted towards POSEIDON group IV (age 35 years or older, AFC <5, and/or AMH <1.2 mg/mL).
Recent studies have shown beneficial reproductive outcomes in normal ovarian responders who underwent dual trigger [16,17]. Also, a randomized study showed that the dual trigger group demonstrated improved clinical pregnancy rates and live birth rates in POSEIDON group IV patients [18]. However, no study has yet reported the clinical pregnancy rate, MII oocyte retrieval rate, and fertilization rate resulting from the difference in the dose of the dual trigger. Hence, we focused on the dose of the hormones used in the dual trigger cycle and whether the two boluses of GnRHa made clinical differences from a single bolus in POSEIDON group IV patients.
Materials and methods
1. Study population
The subjects of this cohort study were recruited from Fertility Center of CHA Gangnam Medical Center, Seoul, South Korea between January 2018 and June 2020. The Institutional Review Board of CHA Gangnam Medical Center approved this study (IRB No. GCI 2020-112-006). Data were collected from the electronic medical records from 1,893 consecutive healthy women who visited Fertility Center of CHA Gangnam Medical Center due to infertility. Patients who were in the final inclusion criteria fulfilled the POSEIDON group IV criteria (age ≥35 years, with AFC <5 and/or AMH <1.2 ng/mL). The following patients were further excluded from the study; 1) patients who did not receive GnRH antagonist protocol, 2) patients with premature ovarian insufficiency, 3) patients with body mass index (BMI) ≥30, and 4) patients with incomplete data. A total of 1,256 patients were selected, and were divided into two groups, one with one ampoule of GnRHa (n=629), and the other with two ampoules of GnRHa (n=627).
2. Protocols of treatment
Patients were randomly selected to use either one or two boluses of GnRHa for the final oocyte maturation in the dual trigger process. Baseline hormone levels were measured, and the number of antral follicles was counted by transvaginal ultrasound (TVS). Within five days of the menstrual cycle, patients received recombinant follicle-stimulating hormone (rFSH; Follitrope Prefilled syringe Inj., LG Chem Ltd., Seoul, Korea; Puregon, Merck Sharp & Dohme Ltd., Kenilworth, NJ, USA; Gonal-F, Merck Ltd., Seoul, Korea), or human menopausal gonadotropin (HP-hMG; Menopur, Ferring Pharmaceuticals Ltd., Seoul, Korea) for COH.
Throughout the IVF cycle, the response of the patient was closely monitored with TVS and subsequent hormone levels. For pituitary suppression, GnRH antagonist (Orgalutran, Hanhwa Pharma Co., Ltd., Seoul, Korea; Cetrorelix, Merck Ltd.; Ganilever, LG Chem Ltd.) daily protocol was used. For the final oocyte maturation, dual trigger of either one bolus or two boluses of GnRHa (Decapeptyl [triptorelin acetate 0.1 mg], Ferring Pharmaceuticals Ltd., Seoul, Korea) combined with recombinant hCG (Ovidrel, Merck Ltd.) were administered.
Depending on the AFC at the menstrual cycle day 2 or 3, FSH 75-225 IU was given, and when the dominant follicle size was at least 10–12 mm, GnRH antagonist was started. If there were only one to two dominant follicle(s), early dual trigger was given when the dominant follicle was at least 16 mm, due to higher risk of premature ovulation. Otherwise, if the premature ovulation risk was low (several dominant follicles present), dual trigger was done when the dominant follicle was at least 18 mm or more.
Oocyte retrieval via transvaginal ultrasound guidance was executed by the treating physician after 34–36 hours of trigger initiation. Fertilization of the retrieved oocytes was performed by conventional IVF or intracytoplasmic sperm injection after denuding of cumulus cells. Depending on the individual (i.e., estimated endometrial thickness at the transfer day), direct fresh embryo transfer ranging from 3 to 5 days or freezing of the oocytes were decided.
Morphological grading of the embryos after culture was done. On day 3, top quality embryos had the following characteristics: four or five blastomeres on day 2 and at least seven blastomeres on day 3 after fertilization; absence of multinucleated blastomeres and <20% of fragments on day 2 and day 3 [19]. Embryo quality on day 5 was assessed according to the criteria of Gardner and Schoolcraft [20]. Good-quality embryos on day 5 were defined as those with a blastocoel equal to or greater than half the volume of the embryo and a good inner cell mass and trophectoderm. Based on the morphological grade, the embryos were categorized as good-quality blastocysts (excellent, good, or average) and poor-quality blastocysts.
3. Outcome measures
The primary outcome of this study was oocyte retrieval rate, and we sought to compare the number of total retrieved oocytes, the number of MII oocytes, oocyte retrieval rate, cancellation rate, fertilization rate, actual embryo transfer rate, transfer rate of at least one top-quality embryo, and clinical pregnancy rate between the two groups as secondary outcomes. Clinical pregnancy rate was defined as the sonographic finding of fetal heartbeat starting from 6 to 7 gestational weeks. Cancellation rate was defined as patients with no viable oocytes or retrieval failure.
4. Statistical analyses
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 26.0 package (IBM, Chicago, IL, USA). Independent t-tests were used for quantitative variables. Standardized coefficients of oocyte retrieval rate and clinical pregnancy rate were analyzed with multiple regressions, with adjustments for confounding factors such as age, BMI, duration of infertility, previous IVF attempts, basal FSH, antral follicle counts, and serum AMH. A value of P<0.05 was considered statistically significant.
Results
Table 1 shows the demographic features of the selected patients fulfilling the POSEIDON group IV who underwent final oocyte maturation with one bolus or two boluses of GnRHa (Triptorelin). No significant differences were found in the baseline characteristics of the two groups.

Baseline characteristics of patients (POSEIDON group IV) with diminished ovarian reserve (DOR) in comparison of one ampoule of two ampoules of dual trigger
Between the two trigger groups, stimulation cycle characteristics show that there were no significant differences in the days of stimulation cycles of the two groups, days of GnRH antagonists used, the total dosage of gonadotropins administered, peak estradiol on the trigger day, endometrial thickness at the embryo transfer day, and cancellation rate, as described in Table 2.
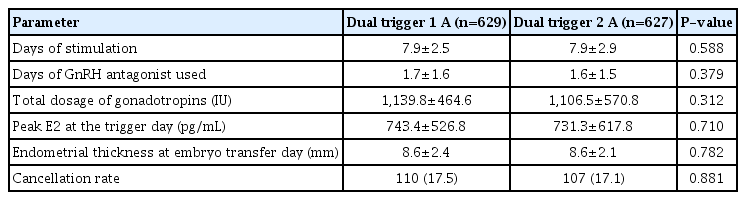
Stimulation cycle characteristics of patients (POSEIDON group IV) with diminished ovarian reserve (DOR) in comparison of one bolus of two boluses of dual trigger
Table 3 compares the retrieval characteristics and pregnancy outcomes of patients between the two groups. The numbers of total retrieved oocytes and MII oocytes, MII retrieval rate and fertilization rate did not show significant differences. However, the percentage of patients who received the transfer of at least one top-quality embryo was significantly lower in the two ampoule group (34.3% vs. 26.0%, P=0.001). Furthermore, oocyte retrieval rate and clinical pregnancy rate were not significantly different between the two groups.

Retrieval characteristics and pregnancy outcomes of patients (POSEIDON group IV) with diminished ovarian reserve (DOR) in comparison of one bolus of two boluses of dual trigger
In Table 4, differences in oocyte retrieval rate and clinical pregnancy rate between the two trigger groups were compared, controlled with several confounding factors. Infertility duration (P=0.012), AMH (P<0.001), basal FSH (P=0.006), and total FSH dose (P=0.006) showed significance as a predictor of oocyte retrieval rate. AMH also significantly affected the clinical pregnancy rate (P=0.037).
Discussion
In the process of final oocyte maturation, dual trigger has been a matter of interest for clinicians over the recent years, and different models have been developed [21]. In the past, overcoming the luteal phase insufficiency caused by GnRHa trigger alone has been the key point for the final oocyte maturation, which came to the advent of dual trigger. A previous study demonstrated that in patients with a higher rate of immature oocytes in the past IVF cycles, dual trigger showed a significantly higher numbers of mature oocytes and embryos transferred and a higher number of top-quality embryos [22]. Also, another study reported that in patients with a history of >25% immature oocytes retrieved in previous IVF cycles, dual trigger for oocyte maturation induced a significantly greater number of mature oocyte retrieval than hCG only [23].
In a molecular sense, several paracrine factors involved in LH surge, amphiregulin (AREG) and epiregulin (EREG) as representatives are known to play key roles in the oocyte maturation and ovulation process [24]. A previous study showed that LH stimulation is known to induce the transient expression of the AREG and EREG, part of the epidermal growth factor (EGF), and these factors result in events such as cumulus expansion and oocyte maturation [25]. Another study showed that after hCG stimulation of mural granulosa cells in-vitro, peak expressions of AREG and EREG mRNA were observed, and mRNA expression levels of AREG were positively correlated with the number of oocytes and top-quality embryos [26].
GnRH agonist used for the final oocyte maturation is known to induce both an FSH surge and an LH surge, which is similar to the LH surge in the natural menstrual cycle, showing a smaller strength and shorter duration than a single hCG cycle [27]. FSH surge is important because FSH is essential in the formation of LH receptors in cumulus granulosa cells in animal studies and is involved in ovulation and oocyte maturation [28,29]. In our study, the single bolus Gn-RHa group showed higher top-quality embryo rates than the double bolus group. A double dosage of GnRHa may overly suppress the FSH and LH hormonal surge, which may result in a decreased top-quality embryo rate, but further investigation is warranted.
Our study presents one of the novel approaches to target the dual trigger itself and divides the group into two groups of a single bolus and two boluses of GnRHa. Our findings show that MII oocyte retrieval rate, fertilization rate, and clinical pregnancy rate did not differ between the two groups. The two boluses of GnRHa showed equivalent or disadvantages over a single bolus, contrary to theoretical thoughts. Also, AMH was a significant predictor of oocyte retrieval rate (standard coefficient [SC], 0.127; P<0.001) and clinical pregnancy rate (SC, 0.070; P=0.037), despite the fact that this study group was comprised of only poor responders with very minimal differences in AMH levels (AMH <1.2 ng/mL). Overall, the results suggest that a single bolus GnRHa showed no significant clinical difference compared to the double bolus in the dual trigger regimen.
The limitations of this study are as follows; 1) patients received various types of gonadotropins, although all cycles were antagonist cycles, providing the possibility of differences in medication responses. 2) This was a retrospective cohort study conducted between January 2018 and June 2020, giving way to a possible chronological bias. 3) The study groups included a small number of endometriosis patients, which may be an influential factor in determining the oocyte quality. However, the two study groups had no statistically significant differences in the number of endometriosis patients and the primary outcomes. Also, the numbers of endometriosis patients in both groups were too low (around 4% each) to perform further subgroup analysis.
We concluded that a single bolus of GnRHa showed comparable clinical results to two boluses in the dual trigger regimen. In all, GnRHa doses in dual trigger should be decided based on individual clinical situations considering cost-effectiveness and patient compliance, but further investigation will be needed.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of CHA Gangnam Medical Center, CHA University. All methods in the research were carried out in accordance with the guidelines and regulations of IRB of CHA Gangnam Medical Center.
Patient consent
All study participants provided informed consent.
Data sharing statement
The datasets generated and analyzed in the current study are not publicly available due to restrictions by CHA University but are available from the corresponding author on reasonable request.
Funding information
Funding information is not applicable, and the authors declare no conflict of interest.

