Development of an endometriosis self-assessment tool for patient
Article information
Abstract
Objective
This study aimed to develop and verify an endometriosis self-assessment tool (ESAT).
Methods
A non-experimental, descriptive, correlational study design was used. Candidate items were developed based on a conceptual framework constructed using the results of in-depth interviews and an integrative literature review. The construct validity of the developed tool was also examined. One-hundred and forty-two participants (117 patients with endometriosis and 25 patients without endometriosis) were included in the validity and reliability tests. The data were collected between August and December 2018. Nomological validity was verified based on significant correlations between the ESAT and the quality-of-life scores.
Results
A 21-item ESAT was developed, and its construct validity was supported. Exploratory factor analysis indicated that the tool consisted of four components (gastrointestinal symptoms, dysmenorrhea, usual symptoms, and the amount and characteristics of menstrual bleeding) with a variance of 61.6%. The variance in quality-of-life scores, as explained by the ESAT scores, was relatively high. Receiver operator characteristics curve analysis indicated that ESAT scores significantly differentiated endometriosis from non-endometriosis with fair discriminatory power at a cut-off score of 50 (sensitivity, 0.76; specificity, 0.72; area under the curve, >0.75; P<0.001). This means that patients with ESAT scores >50 points were more likely to have endometriosis. Thus, the reliability of the ESAT was confirmed.
Conclusion
The devised tool appears valid and reliable. This tool may allow women to determine their risk of endometriosis by distinguishing between normal and pathological menstruation-related symptoms.
Introduction
Endometriosis is a condition in which endometrial tissue grows outside the uterus and induces a local inflammatory response [1,2]. Common symptoms of endometriosis include dysmenorrhea, chronic pelvic pain, menorrhagia, dyspareunia, dysuria, dyschezia, and infertility [3]. There is no etiological explanation for the disease; however, several hypotheses have been proposed [1,2,4]. Furthermore, the chronic nature and severity of pain often leads to a marked deterioration in patient quality of life [5]. Endometriosis may have considerable clinical, economic, and psychological impacts [6]. Endometriosis can affect women of any age from menarche to menopause.
Women with endometriosis often consider chronic pelvic pain as normal and something that should be endured despite its deleterious impact on their quality of life [5,7]. Prentice [8] reported that more than half of patients with endometriosis believed that they had no medical issue of significance before diagnosis. In general, women feel uncomfortable visiting obstetricians and gynecologists, and they are reluctant to disclose problems associated with their periods [9]. Consequently, diagnostic delays are a common problem in endometriosis [7,10]. Laparoscopy provides the only reliable way to definitively diagnose endometriosis; however, it is not routinely performed because it involves surgery [11]. Several endometriosis classification systems have been developed, such as the American Society for Reproductive Medicine classification, the Enzian classification, the American Association of Gynecological Laparoscopists classification, and the classification based on the Endometriosis Fertility Index [12]; however, most were developed for use by health professionals [12,13]. A tool is needed to help patients perceive their symptoms as pathological and to make them feel suspicious of their condition. Few self-assessment tools have been proven to be reliable and reasonable to suspect endometriosis and induce hospital visits, considering that women simply experience severe menstrual pain.
In the context presented above, patient self-assessment can be useful, as it helps women identify pathological symptoms and decide whether they should consider seeking professional advice. Accordingly, we developed a simple, easily applied, and accurate endometriosis self-assessment tool (ESAT) and evaluated its validity and reliability. We emphasize that the ESAT is not a diagnostic tool to be used in hospitals but rather a self-assessment tool that informs women of their likelihood of endometriosis prior to diagnosis. It is hoped that the ESAT might alert women that their symptoms should be taken seriously and that they should consider seeking advice from professional obstetrician/gynecologists (OB/GYN). Being diagnosed by a doctor provides a plan for the future in ambiguous situations for women with endometriosis, and it makes it easy to seek understanding from family and colleagues regarding the problems caused by endometriosis [2,5,10]. This study was conducted to develop and verify an ESAT. The specific aims of this study were 1) to develop a tool based on a series of items identified through in-depth interviews and a literature review, 2) to determine the content and construct validity of the devised tool, and 3) to test its reliability.
Materials and methods
1. Item development
Candidate items were developed based on a conceptual framework constructed using the results of in-depth interviews and an integrative literature review. The content validity of the ESAT was examined.
1) In-depth interviews
In-depth interviews were performed after obtaining permission from the Human Research Committee of Inha University (IRB No: 150415-2A). Recruitment notices containing a brief description of the interview purpose, procedures, and contact information were posted on the bulletin board of an Internet endometriosis self-help group on a Korean portal site (Daum and Naver). Fourteen volunteers diagnosed with endometriosis as determined after laparoscopy by obstetrics and gynecology physicians were recruited. In-depth interviews were conducted between June 2015 and May 2016. Indepth interviews revealed that the most common symptom of endometriosis was pain, particularly severe dysmenorrhea that was not relieved by analgesics, and that the pain occurred around the pelvis, back, lower abdomen, or external genitalia. In addition, mittelschmerz, dysuria, dyspareunia, and dyschezia were also addressed. Pain was also found to occur at various body sites (pelvis, muscles, shoulder, external genitalia, back, or hip joints) at any time, regardless of the menstrual cycle. Heavy, thick, and dark-colored menorrhagia, longer periods, and shortened cycles (even two periods per month) have also been reported.
Gastrointestinal symptoms were common and included abdominal distention or gas accumulation, constipation, diarrhea, irritable bowel syndrome, and indigestion, as were psycho-emotional problems such as anxiety, emotional stress, and poor quality of life. Other symptoms such as fatigue, shivering, lethargy, pollakisuria, nycturia, paresthesia, numbness, and edema have also been reported. Notably, the characteristics and intensities of such symptoms varied over menstrual cycles, indicating that endometriosis symptoms should be evaluated according to the menstrual cycle phase.
2) Integrative literature review
A literature search on the symptoms of endometriosis was conducted between January and February 2017. The primary information sources targeted were PubMed, Elton B. Stephens Company (EBSCO), Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Research Information Sharing Service (RISS). The search terms used were ‘endometriosis’, ‘symptoms’, ‘signs’, and ‘diagnostic criteria’, which were determined by consensus between the authors after several trials. A total of 32 studies were selected based on predetermined inclusion/exclusion criteria (Fig. 1).
The literature review and in-depth interview findings were similar. The most common symptoms were severe dysmenorrhea of the lower abdomen, pelvis, back, external genitalia, muscles, or joints [11,14]. Mittelschmerz syndrome, dyspareunia, dysuria, and dyschezia were also common [14,15]. Menorrhagia, irregular menstruation cycles, altered menstruation patterns, and shortened menstrual cycles have also been reported [11,16–18].
The review showed that gastrointestinal symptoms such as irritable bowel syndrome, abdominal distension, diarrhea, constipation, indigestion, nausea, and vomiting were common [2,3,11,14,16,18–22]. Infertility or difficulty getting pregnant [2,11,15,17,18,20] and psycho-emotional problems such as depression, anxiety, or stress [2,11,22] have also been reported as symptoms of endometriosis.
3) Item development
To develop the candidate items, a conceptual framework of endometriosis symptom experiences was established based on a list of endometriosis symptoms identified by in-depth interviews and a literature review. Symptoms of endometriosis were classified according to menstrual cycle phases: premenstruation, menstruation, and usual. The three phases were defined as follows: 1) pre-menstruation=from the day of ovulation until the onset of menstruation; 2) menstruation during menstrual flow; and 3) usual=any time, regardless of the menstruation cycle. A framework was then developed (Fig. 2), and 47 candidate items were devised based on this framework.
To eliminate unnecessary redundancy and identify the best potential items for the scale, 12 volunteers who were diagnosed with endometriosis following laparoscopy by OB/GYN physicians were invited from an Internet endometriosis self-help group (described in the in-depth interviews section). Participants were asked to mark items that they considered relevant to their experiences. Only items selected by more than four participants were chosen, and as a result, 23 items were extracted (two items with a corrected item-total correlation of <0.30 were later deleted to produce the final 21-item ESAT). Responses to the 23 items were scored using a 4-point Likert scale (‘strongly agree’, ‘agree to some extent’, ‘disagree to some extent’, and ‘do not agree at all’), thus, the maximum possible total score was 84.
The ESAT was originally developed in Korea and its validity and reliability was tested in Korean patients in the present study. This tool was translated from Korean into English by a professional native English-speaking proofreader for publication purposes and to encourage global use. Professors with experience in related research fields confirmed that proofreader-translated items had the same meanings as the original items written in Korean.
4) Content validity testing
The content validity of the 23 items was assessed by an expert panel of five professors with experience in developing self-assessment tools. This expert panel evaluated whether the items were suitable for assessing endometriosis using a 4-point Likert scale: 1) not valid at all, 2) less valid, 3) valid, and 4) very valid. Content validity ratios (CVRs) of the items were computed. Lawshe [23] recommended that a CVR ≥0.99 indicated ‘good’ content validity. In the present study, 22 items had a CVR of 1.0, and one had a CVR of 0.99; the wording of this item was deemed ambiguous by the expert panel and the item was modified. Accordingly, all the 23 items were included in the ESAT.
2. Construct validity testing
To test the construct validity of the 23-item ESAT, exploratory factor analysis, regression analysis (for nomological validity testing), and receiver operator characteristic (ROC) curve analysis were performed.
1) Participants
All patients with endometriosis were treated at OB/GYN outpatient clinics at two branches of a university hospital in Seoul and Bucheon. Potential candidates were nominated by two physicians in the clinic. Patients who satisfied the following criteria were conveniently selected: 1) diagnosis of endometriosis by OB/GYN physicians based on laparoscopy findings; 2) age between 19 and 50 years; 3) ability to read and comprehend the questionnaire; and 4) provision of written consent after being informed of the study purpose and procedures. After applying the selection criteria, 117 patients were included in this study. In addition, 25 non-endometriosis patients who met inclusion criteria numbers 2 to 4 above and were treated at the same clinics for other OB/GYN diseases were included in the ROC curve analysis.
According to the literature, the minimum sample size required for factor analysis is 5–10 subjects per item [24], which in the present study amounted to 115 subjects (23 items×5). For the regression analysis, the power analysis using the G*power program indicated that a minimum sample size of 15 subjects was required (α=0.05, power [1-β]=0.80, effect size=0.63, number of predictors=1). An effect size of 0.63 was chosen based on the findings of Gallagher et al. [25]. For ROC curve analysis, a minimum sample size of 50 subjects (25 positive and negative cases; α=0.05, power [1eb]=0.80, area under curve [AUC]=0.80) was computed using the easyROC program (http://www.biosoft.hacettepe.edu.tr/easyROC). The AUC was 0.80 according to the method described by Nnoaham et al. [26].
The required sample sizes were 115 for factor analysis, 15 for regression analysis, and 50 for ROC curve analysis in the present study. When different sample sizes are calculated for different types of statistical analyses in a study design, the selection of the largest sample is often driven. Therefore, the minimum sample size required for this study was 115. In addition, 25 non-endometriosis patients were recruited for ROC curve analysis.
2) Measures
Nomological validity is a form of construct validity that examines whether relationships among variables are consistent with empirical evidence or theories [27]. In the present study, the relationship between ESAT scores and endometriosis-related quality of life was assessed because endometriosis symptoms have been consistently reported to significantly affect quality of life [7,10,22,28,29]. Endometriosis-related quality of life was assessed using the Endometriosis Health Profile-5 (EHP-5; a short form of EHP-30). The EHP-5 consists of 11 items (5 cores and 6 modular items). The construct validity and reliability of this tool were well supported by the original developers [30].
Data collection was performed after obtaining approval from the human research committee (XC18QEDE0040K) at Catholic Medical Center where the data were collected and from the president and directors of the OB/GYN department of The Catholic University of Korea St. Mary’s Hospital. Data were obtained through a medical record review, and participants completed a self-report questionnaire between August and December 2018. Statistical analyses were performed using SPSS/PC 21 (Datasolution, Seoul, Korea) and easyROC (http://www.biosoft.hacettepe.edu.tr/easyROC).
Results
1. Descriptive statistics of subject characteristics
The mean age of 117 patients was 32.27 years (standard deviation=7.43; <20 years, 0.9% [n=1]; 20–29 years, 17.9% [n=21]; 30–39 years, 41.0% [n=48]; and 40–50 years, 40.2% [n=47]). The mean age of menarche of endometriosis patients was 12.87±1.58 years and their mean menstrual cycle duration was 27.05±7.20 days. Fifty-nine (50.4%) of the patients were married, 48 (41.0%) had previously been pregnant, and 41 (35.0%) had experienced childbirth. Of the 56 (47.9%) patients with a family history of OB/GYN complications, 47 (40.7%) had a family history of uterine problems (uterine myoma [n=47] and endometriosis [n=6]). Thirty-one (26.5%) of the 117 patients engaged in regular exercise with a mean frequency of 2.77±1.13 times per week.
2. Construct validity of the ESAT
1) Exploratory factor analysis
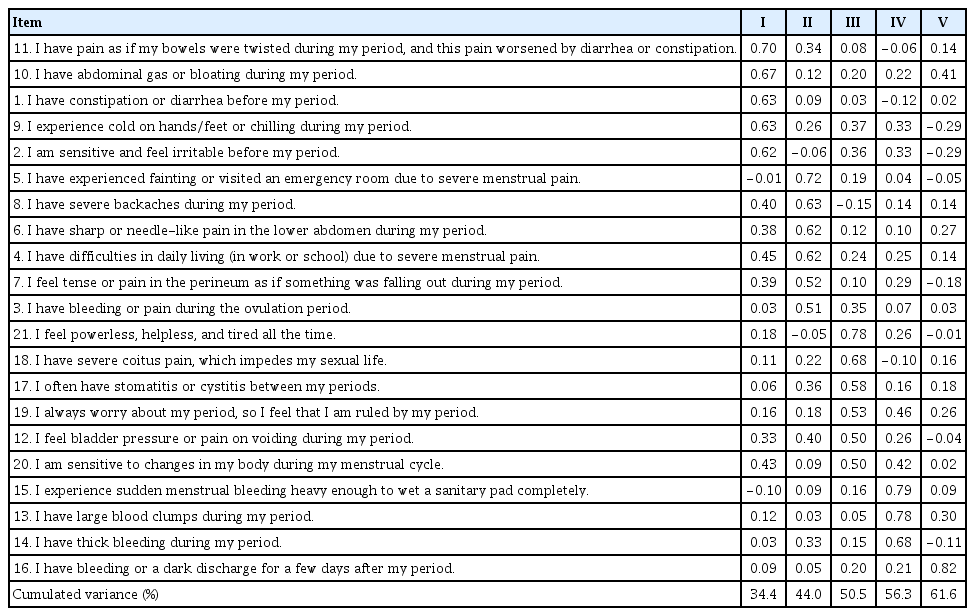
Prior to performing the factor analysis, we assessed the corrected item-total correlations; that is, the correlations between the items and the total ESAT scores. According to the literature, the minimum acceptable correct item-total correlation value of an appropriate item for construct validity is 0.3 [31]. Two items with a corrected item-total correlation of <0.30 were deleted. The remaining 21 items were finally selected and subjected to an exploratory factor analysis.
The Kaiser-Meyer-Olkin (KMO) test was used to evaluate sampling adequacy, and Bartlett’s sphericity test was used to confirm whether the correlation matrix was diagonal (indicating no correlation). According to Kang [31], a KMO value >0.60–0.80 is required for factor analysis, and according to Bartlett’s sphericity test, a significant result (P<0.05) indicates that a correlation matrix is a diagonal correlation matrix. We found a KMO of 0.85 and Bartlett’s sphericity was χ2=1,032.16, P<0.001, indicating factorability. In addition, communality coefficients (an indication of variable usefulness) of the 21 items ranged from 0.40 to 0.77, reaching the minimum requirement of 0.4 [31].
The principal component analysis with a varimax rotation yielded five components with eigenvalues >1. The items were assigned to the components based on their highest loadings. Five items were loaded onto component I (gastrointestinal symptoms), six onto component II (dysmenorrhea), six onto component III (usual symptoms), three onto component IV (amounts and characteristics of menstrual bleeding), and one onto component V (bleeding after the menstrual period). The total variance explained by the five components was 61.6%, that is, 34.4% for component I, 9.6% for component II, 6.6% for component III, 5.8% for component IV, and 5.3% for component V. Because the communality value of the fraction of variance must be ≥0.60 [31], the ESAT appeared to be acceptable in terms of its explanatory power. Notably, only item #16 (“I have bleeding or a dark discharge for a few days after my period”) was loaded onto component V. After careful consideration, we decided to assign this item to component IV (amounts and characteristics of bleeding) because this item seemed to fit into the characteristics of bleeding. Finally, we concluded that the ESAT should consist of four components (Table 1, Supplementary Table 1).
2) Nomological validity
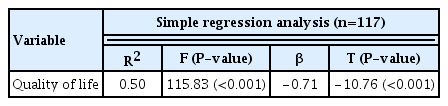
Based on empirical findings that endometriosis significantly affects quality of life [7,22,25,28,29], nomological validity was tested by assessing the correlation between ESAT and quality of life scores in 117 patients with endometriosis. A higher score indicated a higher quality of life. We found that ESAT scores were significantly correlated with the degree of quality of life (β=0.54, P>0.001), which supported the nomological validity of the ESAT. In addition, 50% of the variance in quality of life was explained by the ESAT scores (F=115.83, P<0.001) (Table 2).
3) ROC curve analysis
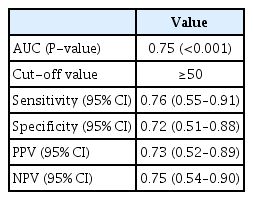
ROC curve analysis was conducted to assess the ability of the ESAT scores to detect the presence of endometriosis. This test was also conducted to determine the optimal cutoff score for the differentiation of endometriosis and non-endometriosis. For ROC curve analysis, 142 patients (117 with endometriosis and 25 without endometriosis) were included in the present study. The 25 endometriosis patients for ROC curve analysis were randomly selected from 117 patients with endometriosis. Data were collected from 50 participants (25 with endometriosis and 25 without endometriosis), and our findings showed that the AUC of the ESAT was 0.75 (P<0.001), which means there was a 75% likelihood of differentiating patients with or without endometriosis. Because an AUC of >0.7 is generally considered suitable for clinical use [32], these findings indicate that the ESAT can be used to effectively detect endometriosis (Fig. 3).
The Youden index was calculated to determine the optimal ESAT cutoff score for endometriosis, which was found to be 50 (sensitivity, 0.76; specificity, 0.72) (Table 3). This means that patients with an ESAT score >50 points were more likely to have endometriosis.
3. Reliability testing
In this study, Cronbach’s alpha of the ESAT was 0.90, and those of its four components ranged from 0.72 to 0.82 (Table 4), indicating ‘good’ reliability despite the small number of items. The corrected item-total correlations of the 21 items ranged from 0.30 to 0.76, which was greater than the minimum acceptable value of 0.30 [31].
Discussion
Endometriosis is prevalent during the reproductive years, with a peak incidence between 30 and 45 years of age [33]. In the present study, the mean age of patients was found to be 37.27 years, which was similar to the findings of Peterson et al. [34]. Unlike the majority of OB/GYN diseases, endometriosis is more common in younger women owing to its hormone-sensitive nature [35].
In this study, a 21-item ESAT was developed. Exploratory factor analysis yielded four components with a variance of 61.6%, supporting the construct validity of the ESAT. These four components were named based on the contents and meanings of the items loaded on the components: gastrointestinal symptoms (component I), dysmenorrhea (component II), usual symptoms (component III), and amounts and characteristics of menstrual bleeding (component IV).
The highest total variance was observed for gastrointestinal symptoms (component I). Empirical evidence also shows that gastrointestinal symptoms, such as irritable bowel syndrome, bloating, diarrhea, constipation, gas, and indigestion, are the most frequent symptoms experienced during or between periods in patients with endometriosis [2,3,11,14,16,19–22]. The dysmenorrhea component exhibited the highest total variance. Dysmenorrhea has detrimental effects on daily life (e.g., sleeping, eating, and moving) and has been found to occur at various body sites in patients with endometriosis [22,28,30,36]. Dysmenorrhea is also known to induce emotional distress, including lethargy, depression, hopelessness, anxiety, isolation, and suicidal ideation [2,8,22,28,36].
Component III consists of the usual symptoms that occur during menstruation or at any other time, regardless of the menstrual cycle. Our in-depth interviews showed that lethargy, fatigue, hypersensitivity to infection, infertility, and dyspareunia were the usual symptoms of endometriosis, and our literature review confirmed that such symptoms commonly occur in women with endometriosis [1,2,11,15,20].
Component IV (amount and characteristics of menstrual bleeding) was found to be the least loaded component. This component consisted of items regarding menorrhagia during periods, thick and dark-colored bleeding, and post-menstrual bleeding [11,16–18].
In the present study, the nomological validity of the ESAT was well supported by significant correlations between ESAT scores and levels of quality of life (QoL); that is, higher ESAT scores were significantly associated with lower QoL scores. The variance in quality of life, as explained by the ESAT scores, was relatively high. Fernandez et al. [37] also showed that endometriosis symptoms, particularly psycho-emotional symptoms caused by infertility or depression, significantly reduce the quality of life of endometriosis patients and their family members.
ROC curve analysis indicated that the ESAT scores significantly discriminated endometriosis with fair discriminatory power, which supported the construct validity of the ESAT. Similarly, other symptom-based endometriosis assessment tools have also been reported to be diagnostically useful, with a probability of correctly classifying endometriosis of approximately 70% [26]. However, it has also been reported that surgical, histological, and ultrasound imaging data, in addition to clinical symptoms, are more effective at detecting endometriosis, and these increased the probability of correctly classifying endometriosis by up to 90% [38]. Our findings indicate that the risk of endometriosis is higher in women with ESAT scores >50 points. However, the ESAT is not a diagnostic tool.
In the present study, a 21-item ESAT was developed, and its construct validity was found to be well supported. Exploratory factor analysis indicated that the ESAT consisted of four components, with a variance of 61.6%. The nomological validity of the ESAT was well supported by significant correlations between the ESAT and quality of life scores, and the variance of quality of life explained by the ESAT scores was relatively high. ROC curve analysis indicated that ESAT scores significantly predicted the presence of endometriosis with fair discriminatory power. The ESAT cutoff score for endometriosis was 50. In addition, the reliability of the ESAT was confirmed. Based on our findings, the ESAT developed in the present study appears to be valid and reliable.
The 21-item ESAT developed during the present study may allow women to self-determine their risk of endometriosis by distinguishing between normal and pathological menstruation-related symptoms and may aid decision-making concerning the advisability of seeking a professional opinion. Women with endometriosis will therefore not hesitate to seek medical intervention.
With respect to study limitations, because the validity and reliability of the ESAT were tested in a relatively small number of patients with endometriosis (n=117), our findings are limited in terms of generalizability. Large-scale, multicenter studies are required to confirm the validity and reliability of the ESAT. In addition, the ESAT needs further verification and refinement as a standardized instrument for endometriosis self-assessment. Despite these limitations, the ESAT could help women adapt to their lives with endometriosis rather than just suffering from uncertain situations.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
In-depth interviews were performed after obtaining permission from the Human Research Committee of Inha University (IRB No: 150415-2A). The study was performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
Written informed consent and the use of images from patients are not required for the publication.
Funding information
None.
Supplementary material
Supplementary Table 1, 2 associated with this article can be found online at https://doi.org/10.5468/ogs.21252.
Supplementary Table 1.
Endometriosis self-assessment tool
Supplementary Table 2.
Systematic review for the symptoms of endometriosis