 |
 |
- Search
| Obstet Gynecol Sci > Volume 66(6); 2023 > Article |
|
Abstract
Cytomegalovirus (CMV) infection during pregnancy is a global silent problem. Additionally, it is the leading cause of congenital infections, non-genetic sensorineural hearing loss, and neurodevelopmental delays in infants. However, this has barely been recognized globally. This condition lacks adequate attention, which is further emphasized by the lack of awareness among healthcare workers and the general population. The impact of CMV infection is often overlooked because of the asymptomatic nature of its presentation in infected pregnant women and newborns, difficulty in diagnosis, and the perception that infants born to women with pre-existing antibodies against CMV have normal neonatal outcomes. This article highlights the latest information on the epidemiology, transmission, clinical manifestations, and development of CMV infection and its management. We reviewed the pathophysiology and clinical manifestations of CMV infection in pregnant women, diagnostic methods, including screening and prognostic markers, and updates in treatment modalities. Current advancements in research on vaccination and hyperimmunoglobulins with worldwide treatment protocols are highlighted.
During pregnancy, cytomegalovirus (CMV) is the most common cause of congenital viral infections, with an incidence of 0.5-1.0% and 0.6-6.0% of all live births in developed [1,2] and developing countries, respectively. The birth prevalence of congenital CMV infection is 0.2-2.5% around the globe and varies by geographic region based on the level of immunity in pregnant women in that population [3]. Additionally, it is the leading non-genetic cause of sensorineural hearing loss (SNHL), a major infection-related cause of congenital malformations in high-income countries and neurological disability [4], and is responsible for approximately 10% of all cerebral palsy and 8-21% of all congenital SNHL cases at birth [5-7].
Globally, the seroprevalence of CMV in people of reproductive age is 86%, with the highest in the World Health Organization Eastern Mediterranean region (90%) and the lowest in European countries (66%) [8]. In areas with high seroprevalence, the rate of congenital CMV is 1-5%; in areas with low seroprevalence, the rate is 0.4-2.0% [3,9-12]. The increasing seroprevalence in most developing countries is due to the high exposure to CMV in overcrowded places in urban settings, increased contact with infants and toddlers, poor hygiene, early sexual debuts, and multiple sexual partners [13]. CMV infection can be primary (exposure to CMV for the first time) or non-primary (reactivation of a latent strain or reinfection with a new strain in an infected mother) [14]. The transmission rate of CMV is directly related to gestational age at infection. No severe congenital infection was observed if the maternal infection occurred after 14 weeks of gestation [15].
Approximately 10-15% of congenitally infected babies are symptomatic at birth and develop neurodevelopmental injuries within the first 3 years. Among them, 50-60% will have severe neurological abnormalities, and 20-30% will die of disseminated intravascular coagulation, hepatic dysfunction, or bacterial sepsis. Within the asymptomatic group (85-90% of cases), approximately 5-15% will develop late sequelae of CMV, such as SNHL, delayed psychomotor development, and visual impairment [16,17]. Kagan and Hamprecht [18] concluded that the overall risk of symptomatic congenital CMV (cCMV) infection after maternal seroconversion in the first trimester is 1 in 10. It then changes to 1 in 4 if amniocentesis is positive for CMV and 1 in 200 if negative. Among positive amniocentesis cases, in the presence of cCMV ultrasound findings, the risk increases to 1 in 2; in the absence, it decreases to 1 in 8 [18].
CMV infection remains a crucial challenge worldwide, without actual therapy for pregnant women and established vaccines, and there is confusion regarding its pathophysiology, prevention, and treatment [19]. Due to its prevalence in the general population, uncertainty about its transmission and the asymptomatic nature of the disease in healthy women pose a threat to the prevention and treatment of CMV infections [13]. Finer elucidation of the factors that influence the occurrence of congenital infections and diseases in the offspring of immune mothers is required to plan preventive strategies and estimate the potential effectiveness of vaccines [20].
CMV is a double-stranded DNA virus that belongs to the Herpesviridae family. The virus is a large, complex agent with genetic material 20 times larger than that of the human immunodeficiency virus. Human CMV infections result from the virus-mediated evasion of T-cell immunity. The CMV lyses host cells, infects various cells and tissues, and remains latent within the host. The virus disseminates throughout the body by infecting the white blood cells and vascular endothelial cells. They interfere with the recognition and processing of viral proteins by major histocompatibility complex (MHC) class I molecules, induce abrupt helper T-cell responses by degrading MHC class II molecules and inhibit the action of natural killer cells in the host [21].
CMV spreads from the placenta to developing embryos or fetuses. They are transmitted via the syncytiotrophoblast layer covering the chorionic villi, resulting in unfavorable placental and consequent fetal development. Additionally, trophoblast infection impairs interstitial implantation, which explains the early pregnancy loss associated with CMV infection [22].
The most common route of CMV spread is through the saliva or other bodily secretions of toddlers who shed the virus asymptomatically for months. In addition to transplacental transmission, breast milk can spread the virus to infants, particularly those born preterm. CMV can be transmitted sexually through solid organs or hematopoietic cell transplantation and rarely through blood transfusion from seropositive patients [23]. The transmission rate via breastfeeding is 58-69% in term babies, 5.7-58.6% in preterm infants, and 38% in preterm babies with a birth weight <1,200 g or gestational age <32 weeks [24,25].
The risk of vertical transmission and unfavorable fetal outcomes is highest with maternal primary CMV infection. However, the risk of transmission can be determined weeks before conception [4]. The rate of transmission is directly related to the gestational age at the time of maternal infection, being lowest in the first trimester (30%), followed by the second trimester (45%), and highest in the third trimester (72%) [26]. The rate of CMV transmission is 0-10% during the preconception period (2-8 weeks before the start of the last menstrual period [LMP]), while it is approximately 25-45% during the periconceptional period (1 week before to 5 weeks after the LMP) [27]. Approximately 30-35% of vertical transmission occurs following primary infection, and 10-15% of congenital diseases. Hamilton et al. [28] reported CMV infection modulates the placental immune environment suggesting CMV-induced cytokines such as monocyte chemoattractant protein 1 and tumor necrosis factor alpha might initiate placental damage (Fig. 1). Transmission during non-primary infections can be as low as 1.2% [29]. Despite the highest transmission rate in the third trimester, fetal outcomes are always good [30]. Primary CMV infection in the third trimester or at the delivery time is not contraindicated for vaginal delivery [31]. Of the asymptomatic cases at birth due to primary infection, 25% develop sequelae by 2 years, while 8% of non-primary CMV infections develop sequelae by 2 years and 14% by 5 years of age [32].
The role of natural defense mechanisms against the reinfection and transmission of congenital infections is not widely understood. However, pre-existing immunity to CMV infection is protective against new CMV infections [33]. The risk of congenital CMV infection is 69% lower in infants born to seropositive mothers before pregnancy than in those who undergo seroconversion during pregnancy [20]. Breastfeeding with CMV infection is recommended if the gestational age is >32 weeks at birth or if the birth weight of the neonates is >1,000 g. Breast milk, including colostrum, should be pasteurized for preterm babies after 3 days of lactation [34]. A detailed classification of congenital infections is presented in Table 1.
Non-primary CMV infection was defined as CMV infection before pregnancy. Similar to other herpes viruses, CMV remains latent in the host after infection. These are recurrent or secondary infections due to the reactivation of latent infection or reinfection with a fresh strain [35]. Uncertainty remains regarding the epidemiology of non-primary infections. Moreover, deciphering how many pregnant women will experience reactivation or reinfection of CMV, how many will develop congenital infections, or the clinical spectrum of congenital infection following non-primary CMV infection during pregnancy remains a challenge [36]. When anti-CMV antibodies are produced following a primary infection, they do not protect against reactivation or reinfection by a new strain. Hence, seropositivity before pregnancy does not eliminate the risk of congenital non-primary infections [1].
Ultrasound abnormalities can be observed for at least 12 weeks after maternal CMV infection [27]. After confirmation of cCMV infection through amniocentesis, serial ultrasonography is performed every 2-3 weeks until delivery [32]. The involvement of the central nervous system (CNS) in cCMV infection is more destructive than that of other organ systems and is considered irreversible. Neurological deficits are observed at birth or in early childhood and persist throughout life [37]. Various CNS abnormalities associated with cCMV infection are caused by early fetal brain inflammation, destruction, or obstruction. These include ventriculomegaly (>15 mm), microcephaly (<2 standard deviation), periventricular echogenicity, hydrocephaly, increased cisterna magna width (>8 mm), vermian hypoplasia, periventricular cysts, corpus callosum agenesis, lissencephaly, and porencephaly. Ventriculomegaly and microcephaly are associated with poor fetal outcomes. Milder CNS abnormalities include ventriculomegaly (10-15 mm), intracranial calcifications, choroid plexus cysts, subependymal cysts, and intraventricular synechia [38,39]. Involvement of the gastrointestinal system can be indicated by hyperechogenic bowel disease, hepatosplenomegaly, pleural effusion, ascites, or hydrops. Splenomegaly is a common ultrasound finding in cCMV infections, and splenic artery Doppler is a non-invasive marker of congenital infection due to diminished arterial blood flow [40-42]. CMV interferes with cardiac development and causes cardiomegaly, pericardial effusion, and calcifications [43].
Commonly observed symptoms of cCMV infection at birth include jaundice, skin lesions, fetal growth restriction, microcephaly, hepatosplenomegaly, thrombocytopenia, and impaired hearing. CMV-associated SNHL usually presents at birth or progresses and rapidly worsens during the first few years of life [44]. Viral infections lead to labyrinthitis, destruction of the vestibular endolymphatic system and organs (the utricle and saccule), and collapse of the saccular membrane [37]. The risk of hearing loss is associated with the viral load. An increase in viral load and duration of viral urinary excretion in symptomatic and non-symptomatic congenitally infected children is associated with hearing loss [34]. Tables 2,3 summarize the clinical features and ultrasound findings of cCMV infection, respectively.
The most common type of SNHL is a flat hearing configuration (equal degree of hearing loss for all frequencies, high or low), which represents damage to the entire cochlea [45,46]. Progressive SNHL is a sensorineural decrease in hearing of 10 dB at any frequency or auditory brainstem response threshold documented in two separate evaluations. The fluctuating type of SNHL is observed in 20-30% of cases and is defined as a decrease in hearing greater than 10 dB or more at any frequency, followed by an improvement of greater than 10 dB at one or more times. Hearing improvements can be observed in otherwise asymptomatic patients [47]. Only 5.7% of congenitally infected neonates, 29.3-44.4% of symptomatic neonates, and 1.6-3.4% of asymptomatic neonates require hearing aids or cochlear implants [48,49]. Neurological sequelae, apart from SNHL, following congenital CMV infection can result in developmental delays, cognitive impairment, cerebral palsy, epilepsy, impaired vision, and autism spectrum disorder [4].
Predicting the prognosis of congenital CMV infection is challenging and depends on three factors: timing of the infection, presence and type of fetal abnormalities, and laboratory markers. Fetal cerebral abnormalities are primary ultrasound prognostic markers [31]. The addition of fetal magnetic resonance imaging to ultrasound was reported to be mutually complementary rather than exclusive to each other for the diagnosis of cerebral lesions, with both specificity and negative predictive value of 93.3% for this combined imaging modality [50,51]. A high viral load in the amniotic fluid, thrombocytopenia, and high DNAemia in the fetus are associated with CMV infection, even in the absence of cerebral anomalies on ultrasound [52].
Walker et al. [53] suggested several screening approaches that could have been effective: 1) to screen all women preconceptionally or early in pregnancy; 2) to screen only women who are at the highest risk of exposure to CMV infection (prolonged contact with toddlers or those who work at daycare centers); 3) “once off” screening around 20 weeks of gestation, including avidity tests with serology, to identify all women who have acquired primary infections early in pregnancy; or 4) targeted screening of pregnant women using ultrasound scans suggestive of congenital fetal infection [53].
Currently, routine screening for CMV is not encouraged because of the lack of effective maternal treatment or counseling programs, epidemiological data regarding the rate of secondary maternal infection and its consequences, difficulty in establishing the prognosis of congenital infection, and possible negative effects of screening, such as increased anxiety, early elective abortion, and abusive amniocentesis [7]. The inclusion of a routine screening test for CMV is beneficial, as it is a major public health problem with significant morbidity and mortality and is more common than Down syndrome. Early screening and reduction of primary infections in mothers are always beneficial, as CMV has no available treatment [54,55]. Reichman et al. [56] hypothesized that pre-conceptional screening of women undergoing fertility treatment would be advantageous in reducing CMV exposure during pregnancy. Notably, none of the international guidelines recommends routine screening for CMV infection in pregnant women.
CMV testing is usually performed in patients with (i) maternal flu-like symptoms (which are primarily nonspecific, such as rhinitis, pharyngitis, myalgia, arthralgia, headache, or fatigue) or those with symptoms of glandular fever (fever, malaise, myalgia, or cervical lymphadenopathy) and negative results for Epstein-Barr virus infection; ii) symptoms of hepatitis (negative tests for hepatitis A, B, and C) during pregnancy; or iii) ultrasound findings suggestive of possible CMV infection [27].
The standard method for diagnosing primary CMV infection is to demonstrate CMV-specific IgG seroconversion in pregnant women who were seronegative for immunoglobulin G (IgG) before pregnancy (Fig. 2). This test is possible if a serum sample is available from a woman before pregnancy. In the absence of a serum sample, the measurement of immunoglobulin M (IgM) and IgG levels is necessary for diagnosis. Although the sensitivity of IgM screening tests is high, they have low specificity for the diagnosis of primary infections. In addition, IgM remains positive for more than a year after an acute infection, as observed in the reactivation or reinfection of CMV with a different strain. Cross-reactivity with other diseases, such as Parvovirus B19, Epstein-Barr virus infection, or systemic lupus erythematosus, gives false-positive results, making it difficult to diagnose primary infections and differentiate between primary and non-primary infections upon documentation of recent seroconversion [31].
The avidity IgG assay can detect primary infections more accurately than an IgM assay alone. Avidity levels are typically expressed as an avidity index, the percentage of IgG bound to an antigen after treatment with a denaturing agent. Avidity indices (vitek immunodiagnostic assay system CMV IgG index) can be classified into three categories: low (<20%), indicative of a recent primary infection within the last 3 months; high (>80%), excluding a recent primary infection; and intermediate (20-80%), which does not allow discrimination between recent and past infections [57,58]. The Society of Maternal-Fetal Medicine recommends that the diagnosis of primary maternal infection should be based on IgG seroconversion, positive CMV IgM, positive IgG, or low IgG avidity [32]. Other tests available for diagnosing primary maternal infections include a four-fold rise in serum IgG levels over 14-21 days [59]. Fig. 2 shows the maternal CMV serological test results.
The most common ultrasound findings for congenital CMV infection are echogenic fetal bowel, cerebral ventriculomegaly and calcifications, and fetal growth restriction [60,61]. However, it takes 6-8 weeks for the fetus to excrete the virus in the urine after maternal CMV infection. Recognition of this lag interval is necessary for the timing of amniocentesis and to avoid false-negative results. Therefore, amniocentesis is performed at approximately 21 weeks and at least 8 weeks after maternal infection and is the best prenatal diagnostic test for fetal congenital CMV infection. CMV DNA polymerase chain reaction (PCR) is the standard method for detecting infections and nearly excludes antenatal fetal infections. A negative result has a sensitivity of 90-95% and a specificity of 97-100% [62-64]. Cordocentesis is another diagnostic option with the same accuracy as amniocentesis but with more side effects because of the invasiveness of the procedure [62,63].
In newborns, congenital CMV is suspected when: i) there are signs and symptoms consistent with cCMV disease, like microcephaly, being small for gestational age, thrombocytopenia, hepatosplenomegaly, and jaundice (direct hyperbilirubinemia) at birth that cannot be explained by other causes; ii) abnormal neuroimaging findings suggestive of CMV, including periventricular calcifications, lenticulostriate vasculopathy, white matter disease, ventriculomegaly, migrational abnormalities; iii) documented SNHL at birth; and iv) severely immunocompromised newborns upon screening (abnormal T cell excision circles) [65].
CMV infection in neonates should be diagnosed within 21 days of life by extracting CMV from saliva or urine, as it is difficult to distinguish between congenital and postnatal infections after 3 weeks of life. False-positive results with saliva samples have been reported; hence, any positive saliva test should be confirmed by detecting CMV in urine [66]. Virus isolation via culture is expensive, time-consuming, and uncontrollable and cannot be used for screening a larger population. Thus, it is preferable to use a real-time PCR test for diagnosis because it is inexpensive, not influenced by storage and transportation conditions, and requires minimal maintenance [34]. Serological studies in neonates are sensitive, as maternal antibodies remain in circulation for up to 1 year after birth [65]. There is a role for dried blood spot (Guthrie cards) PCR in testing for CMV, but it has low sensitivity and is mainly used in children with delayed-onset sequelae and for retrospective diagnoses. After 3 weeks of age, the diagnosis of CMV infection is based on clinical findings and laboratory testing for CMV, excluding the etiologies of the differential diagnoses of CMV infection [21].
After confirming a CMV infection, infants with congenital infections should be further evaluated using comprehensive physical (weight, height, and head circumference to rule out microcephaly), neurological, and neurodevelopmental examinations. Complete blood counts, liver and kidney function tests, hearing evaluations using auditory brainstem-evoked responses, ophthalmological evaluations, and neuroimaging have been performed [67].
CMV elicits humoral and T-cell responses [68]. Neutralizing antibodies against envelope glycoproteins and viral proteins such as pp65, IE1, and IE2 are involved in the regulation of CMV infection and reactivation [69]. Pentameric (gH/gL/UL128/UL130/UL131A) and glycoprotein B (gB) antibodies neutralize the infection of endothelial, epithelial, myeloid cells, and fibroblasts, respectively [70,71]. Fu et al. [72] speculated that a whole-virus vaccine could induce immune responses similar to those induced by natural infections.
Two previously reported vaccines, the live attenuated virus Towne and recombinant gB/MF59 vaccines, have shown moderate efficacy against CMV in seronegative women. However, both vaccines failed to produce neutralizing antibodies against the pentameric complex, a response similar to that observed in natural CMV infections [73-75]. The V160 vaccine, derived from the live AD169 strain, which was genetically engineered to express the pentameric complex, has been evaluated in phase I trials for vaccine safety and immunogenicity in CMV-seropositive and -seronegative women and has exhibited an acceptable safety profile [33]. However, uncertainty regarding the ability of naturally acquired immunity to prevent future infections is a major impediment to the development of vaccines against CMV infections. These vaccines should protect against primary infections, reactivation, and reinfection with different CMV strains [20].
The do’s and don’ts practices to mitigate the spread of CMV infection are as follows: the dos include assuming that children below 3 years of age shed CMV in their urine and saliva, improvements in personal hygiene practices and thorough hand washing is needed after contact with young children’s dirty laundry, diaper changes, wiping runny nose or drool, or handling toys, pacifiers, or toothbrushes. The don’ts include contact with saliva when kissing a child; sharing food, utensils, drinks, or toys used by toddlers. This behavioral modification is the most practical and economical way to mitigate the risk of CMV infection during pregnancy. Leruez-Ville et al. [4] reviewed the benefits of preventive behaviors by both parents. They avoid contact with bodily fluids from infected individuals, especially toddlers, from preconception to 14 weeks of gestation. Furthermore, Barber et al. [76] emphasized the need for effective interventions in routine care to change patient behavior and reduce the risk of CMV infection.
CMV hyperimmunoglobulin (CMV HIG) is not indicated to prevent the fetal transmission of maternal primary CMV infections. Moreover, antiviral treatment for fetal CMV infection is not routinely recommended [67] (Fig. 1).
The proposed antiviral drugs in the management of CMV infection are foscarent, cidofovir, ganciclovir and valaciclovir. They are being used in the treatment of non-pregnant, immunocompromised and transplant patients. All except, Valaciclovir, have teratogenic and toxic effects, thus not recommended during pregnancy. Valacyclovir, an oral prodrug of acyclovir with high bioavailability, has been used to treat congenital CMV infections [37,77,78]. Jacquemard et al. [77] were the first to report the use of oral valaciclovir during pregnancy to treat known fetal infections. They achieved therapeutic drug concentrations in maternal and fetal blood, thereby decreasing the viral load in fetal blood. However, the study lacked randomization [77]. The phase II open-label trial CYMEVAL (in utero treatment of CMV congenital infection with valaciclovir) used a high dose of oral valaciclovir (8 g/day) in women with moderately to severely infected fetuses with non-severe ultrasound features. The trial showed that 82% of fetuses were born asymptomatic in the treatment group and 43% in the placebo group. This study further emphasizes the safety profile of high-dose valaciclovir. The only limitation was the lack of randomization [47].
Shahar-Nissan et al. [79] were the first to study the use of valaciclovir to prevent fetal transmission following maternal primary CMV infection. The treatment group received 8 g/day valaciclovir (16 capsules per day: 8 capsules in the morning and 8 in the evening; each capsule contained 500 mg valaciclovir and 200 mg inactive ingredients), whereas the control group received the same amount of identical placebo tablets. Of the 44 fetuses in the valaciclovir group, only 3 were symptomatic; 14 out of 43 fetuses in the control group were symptomatic [79]. A case-control study by Faure-Bardon et al. [80] confirmed the use of valaciclovir in the first trimester of pregnancy for secondary prevention of cCMV in a well-established routine maternal serum screening policy. Similarly, in a retrospective study, Egloff et al. [81] reported that valaciclovir reduced maternal-fetal transmission in the second trimester, especially in the presence of positive maternal viremia. A non-randomized, first-of-its-kind study evaluated the efficacy of the combined use of valaciclovir (2 g/6 hours) and CMV HIG (200 international unit [IU]/kg) for the treatment of cCMV, especially in the presence of changes in cerebral echography or high viral load in the amniotic fluid [82].
Despite numerous studies, there is still no approved treatment for CMV infections during pregnancy [83]. However, with accumulating evidence reporting the benefit of high-dose valaciclovir, Codaccioni et al. [84] used off-label valaciclovir (8 g/day) in infected pregnant mothers and reported a low maternal viral load and the birth of a clinically asymptomatic infant. Table 4 lists the international guidelines and recommendations for the management of cCMV infections.
Nigro et al. [85] were the first to use CMV HIG to prevent maternal-fetal transmission and congenital diseases in infected fetuses. A phase II, randomized, double-blind study evaluated the efficacy of hyperimmunoglobulin and showed that, among 123 subjects, the rate of congenital infection in the hyperimmunoglobulin group was 30% compared to 44% in the placebo group. No significant differences were observed between or within the two groups regarding viral transmission, levels of virus-specific antibodies, T cell-mediated immune responses, or viral DNA load. No significant modification was noted in the primary infection during pregnancy [86]. In a non-randomized trial, Kagan et al. [87] studied the biweekly use of CMV HIG (200 IU/kg) in seropositive women (primary infection) in the first trimester until 20 weeks of gestation to prevent maternal-fetal transmission. Recently, a multicenter, double-blind trial concluded that the administration of CMV HIG before 24 weeks of gestation did not lower the incidence of congenital infection or perinatal death compared to the placebo group [88]. In an ongoing observational study, CMV HIG was reported to be beneficial for treating primary infections in the first trimester or periconception period when administered biweekly at a dose of 200 IU/kg [89]. Given the ambiguity of the results of HIG administration, it is currently not recommended for treatment [31].
A newer drug, letermovir, can be used for the prophylaxis of CMV infection in seropositive recipients of allogeneic hematopoietic stem cell transplants aged over 18 years. However, further studies are required to determine their use in the prevention and treatment of congenital CMV infections during pregnancy [4]. Letermovir, an antiviral that targets the viral terminase complex, was approved by the United States Food and Drug Administration in 2017 as a prophylactic agent to prevent CMV infection and disease in hematopoietic stem cell transplant recipients. Hamilton et al. [90] studied Letermovir, Maribavir, and other antivirals in human placental models and ex vivo placental tissue to assess their antiviral efficacy and toxicity and found that letermovir exhibited effectiveness and low toxicity in placental trophoblast cell cultures during the first trimester and placental explant histo-cultures during the third trimester. However, further research is needed to characterize these antivirals in terms of their applicability to extended antiviral treatment regimens. In a secondary analysis of a multicenter randomized study of CMV HIG to prevent cCMV infection, models were developed to predict cCMV infection in the presence of primary maternal infection and the absence of ultrasound findings. The four-factor model included government-assisted medical insurance, IgG avidity <32%, IgM antibody index >4.5 (or high), and CMV detectable in the maternal plasma [91].
CMV infections during pregnancy are a public concern. Women need to be protected from acquiring the disease by following hygienic practices, preventing maternal-fetal viral transmission in utero, and treating congenital infections if they occur. Despite our knowledge of primary CMV infections, we do not fully understand the biology, transmission, and accuracy of tests to diagnose non-primary CMV infections. The roles of antiviral drugs, CMV HIG, and anti-CMV vaccines are still being researched. Randomized trials with larger cohorts are needed to prove the efficacy of antiviral drugs in the treatment and prevention of cCMV infection.
Fig. 1
Transmission mechanisms and treatment protocols for CMV infection during pregnancy. CMV, cytomegalovirus; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor alpha; IUGR, intrauterine growth restriction; IgG, immunoglobulin G; IgM, immunoglobulin M; RT-PCR, reverse transcription polymerase chain reaction; MRI, magnetic resonance imaging; CNS, central nervous system.

Fig. 2
Flow chart for the interpretation of the maternal cytomegalovirus (CMV) serology test results. IgG, immunoglobulin G; IgM, immunoglobulin M.
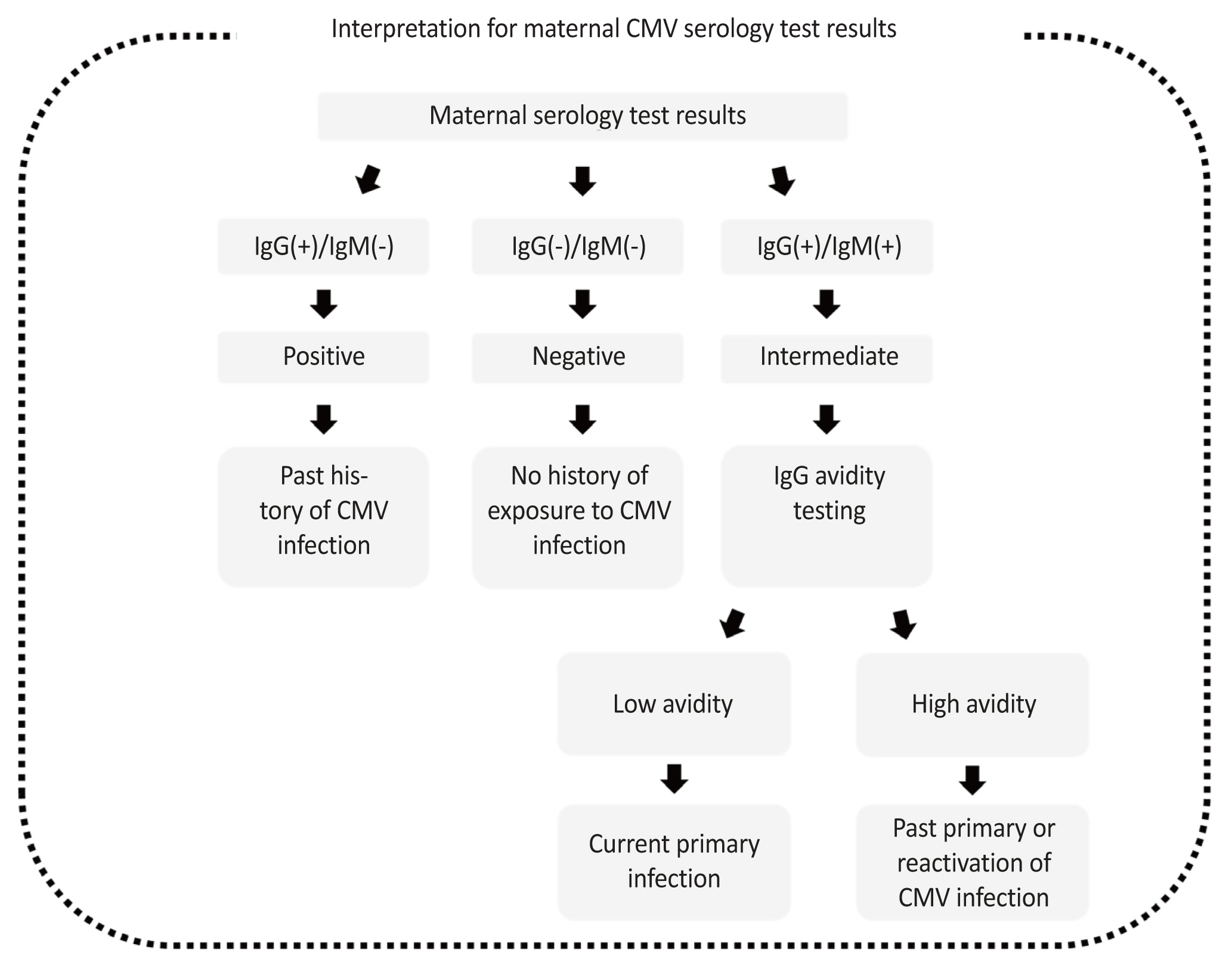
Table 1
Table 2
Signs and symptoms of congenital cytomegalovirus (CMV) infections
Table 3
Ultrasound findings or soft markers of central nervous system involvement in cytomegalovirus (CMV) infection [39]
Table 4
List of guidelines and their recommendations on the management of congenital cytomegalovirus (CMV) infection
| Guideline | High-dose of valaciclovir | High-dose CMV hyperimmune globulin |
|---|---|---|
| ACOG [92] | Not recommended in routine clinical care | Not recommended outside research protocol |
| RCOG [93] | A larger RCT required to test its efficacy to reduce the risk of congenital symptomatic infection | Reserved for research setting |
| SMFM [32] | Only for research purpose (good practice) | Research only (good practice) |
| ISUOG [27] | Lack of RCT, should be used only for research purpose (good practice) | Not a part of clinical care, used only for research purpose (grade B) |
| International congenital CMV recommendation group [67] | Only in moderate to severely infected neonates (level 1) | Lack of RCT, should be used only for research purpose (good practice) |
| Routine antiviral therapy in not recommended (level 2) |
References
1. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007;17:355-63.


2. Stagno S. Cytomegalovirus infection: a pediatrician’s perspective. Curr Probl Pediatr 1986;16:629-67.


3. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17:253-76.


4. Leruez-Ville M, Foulon I, Pass R, Ville Y. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol 2020;223:330-49.


5. Korver AM, de Vries JJ, Konings S, de Jong JW, Dekker FW, Vossen AC, et al. DECIBEL study: congenital cytomegalovirus infection in young children with permanent bilateral hearing impairment in the Netherlands. J Clin Virol 2009;46(Suppl 4):S27-31.

6. Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014;134:972-82.



7. Avettand-Fenoël V, Marlin S, Vauloup-Fellous C, Loundon N, François M, Couloigner V, et al. Congenital cytomegalovirus is the second most frequent cause of bilateral hearing loss in young French children. J Pediatr 2013;162:593-9.


8. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009;49:522-8.


9. Cherry J, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ. Feigin and cherry’s textbook of pediatric infectious diseases e-book: 2-volume set. 7th ed. Amsterdam: Elsevier Health Sciences; 2013.
10. Waters A, Jennings K, Fitzpatrick E, Coughlan S, Molloy EJ, De Gascun CF, et al. Incidence of congenital cytomegalovirus infection in Ireland: implications for screening and diagnosis. J Clin Virol 2014;59:156-60.


11. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20:202-13.



12. Istas AS, Demmler GJ, Dobbins JG, Stewart JA. Surveillance for congenital cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clin Infect Dis 1995;20:665-70.


13. Damato EG, Winnen CW. Cytomegalovirus infection: perinatal implications. J Obstet Gynecol Neonatal Nurs 2002;31:86-92.


14. Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 2011;52:e11-3.


15. Picone O, Vauloup-Fellous C, Cordier AG, Guitton S, Senat MV, Fuchs F, et al. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 2013;33:751-8.


16. Yinon Y, Farine D, Yudin MH. No. 240-cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can 2018;40:e134-41.

17. Gaytant MA, Steegers EA, Semmekrot BA, Merkus HM, Galama JM. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet Gynecol Surv 2002;57:245-56.


18. Kagan KO, Hamprecht K. Cytomegalovirus infection in pregnancy. Arch Gynecol Obstet 2017;296:15-26.



19. Rawlinson WD, Hamilton ST, van Zuylen WJ. Update on treatment of cytomegalovirus infection in pregnancy and of the newborn with congenital cytomegalovirus. Curr Opin Infect Dis 2016;29:615-24.


20. Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003;289:1008-11.


22. Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 2000;74:6808-20.




23. Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis 2001;33:1998-2003.


24. Jim WT, Chiu NC, Ho CS, Shu CH, Chang JH, Hung HY, et al. Outcome of preterm infants with postnatal cytomegalovirus infection via breast milk: a two-year prospective follow-up study. Medicine (Baltimore) 2015;94:e1835.


25. Kurath S, Halwachs-Baumann G, Müller W, Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect 2010;16:1172-8.


26. Enders G, Daiminger A, Bäder U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 2011;52:244-6.


27. Khalil A, Sotiriadis A, Chaoui R, da Silva Costa F, D’Antonio F, Heath PT, et al. ISUOG practice guidelines: role of ultrasound in congenital infection. Ultrasound Obstet Gynecol 2020;56:128-51.

28. Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLos One 2012;7:e52899.



30. Feldman B, Yinon Y, Tepperberg Oikawa M, Yoeli R, Schiff E, Lipitz S. Pregestational, periconceptional, and gestational primary maternal cytomegalovirus infection: prenatal diagnosis in 508 pregnancies. Am J Obstet Gynecol 2011;205:342.e1-6.


31. Navti OB, Al-Belushi M, Konje JC. Cytomegalovirus infection in pregnancy - an update. Eur J Obstet Gynecol Reprod Biol 2021;258:216-22.


32. Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol 2016;214:B5-11.


33. Adler SP, Lewis N, Conlon A, Christiansen MP, Al-Ibrahim M, Rupp R, et al. V160-001 study group. Phase 1 clinical trial of a conditionally replication-defective human cytomegalovirus (CMV) vaccine in CMV-seronegative subjects. J Infect Dis 2019;220:411-9.

34. Chiopris G, Veronese P, Cusenza F, Procaccianti M, Perrone S, Daccò V, et al. Congenital cytomegalovirus infection: update on diagnosis and treatment. Microorganisms 2020;8:1516.



35. Zelini P, d’Angelo P, De Cicco M, Achille C, Sarasini A, Fiorina L, et al. Human cytomegalovirus non-primary infection during pregnancy: antibody response, risk factors and newborn outcome. Clin Microbiol Infect 2022;28:1375-81.


36. Picone O, Grangeot-Keros L, Senat M, Fuchs F, Bouthry E, Ayoubi J, et al. Cytomegalovirus non-primary infection during pregnancy. Can serology help with diagnosis? J Matern Fetal Neonatal Med 2017;30:224-7.


37. Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 2009;22:99-126.




38. Blázquez-Gamero D, Soriano-Ramos M, Martínez de Aragón A, Baquero-Artigao F, Frick MA, Noguera-Julian A, et al. Role of magnetic resonance imaging and cranial ultrasonography in congenital cytomegalovirus infection. Pediatr Infect Dis J 2019;38:1131-7.


39. Cho GJ, Oh MJ, Chung JW, Chung OS, Lee JK, Hur JY, et al. A case of congenital cytomegalovirus infection with severe hydrocephalus in prenatal ultrasonography. Korean J Obstet Gynecol 2004;2224-8.
40. Hawkins-Villarreal A, Moreno-Espinosa AL, Martinez-Portilla RJ, Castillo K, Hahner N, Nakaki A, et al. Fetal liver volume assessment using magnetic resonance imaging in fetuses with cytomegalovirus infection†. Front Med (Lausanne) 2022;9:889976.



41. Gabrielli L, Bonasoni MP, Chiereghin A, Piccirilli G, Borgatti EC, Simonazzi G, et al. Pathophysiology of hyperechogenic bowel in congenitally human cytomegalovirus infected fetuses. Microorganisms 2020;8:779.



42. Prodan N, Sonek J, Wagner P, Hoopmann M, Abele H, Hamprecht K, et al. Splenic artery blood flow as a potential marker for materno-fetal transmission of a primary CMV infection. Arch Gynecol Obstet 2019;299:1289-94.



43. Boucoiran I, Yudin M, Poliquin V, Caddy S, Gantt S, Castillo E. Guideline no. 420: cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can 2021;43:893-908.


44. Marsico C, Kimberlin DW. Congenital cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Ital J Pediatr 2017;43:38.




45. Foulon I, Vleurinck L, Kerkhofs K, Gordts F. Hearing configuration in children with cCMV infection and proposal of a flow chart for hearing evaluation. Int J Audiol 2015;54:714-9.


46. Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 2014;18:2331216514541361.




47. Foulon I, Naessens A, Faron G, Foulon W, Jansen AC, Gordts F. Hearing thresholds in children with a congenital CMV infection: a prospective study. Int J Pediatr Otorhinolaryngol 2012;76:712-7.


48. Goderis J, Keymeulen A, Smets K, Van Hoecke H, De Leenheer E, Boudewyns A, et al. Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. J Pediatr 2016;172:110-5.e2.


49. Foulon I, De Brucker Y, Buyl R, Lichtert E, Verbruggen K, Piérard D, et al. Hearing loss with congenital cytomegalovirus infection. Pediatrics 2019;144:e20183095.



50. Benoist G, Salomon LJ, Mohlo M, Suarez B, Jacquemard F, Ville Y. Cytomegalovirus-related fetal brain lesions: comparison between targeted ultrasound examination and magnetic resonance imaging. Ultrasound Obstet Gynecol 2008;32:900-5.


51. Picone O, Simon I, Benachi A, Brunelle F, Sonigo P. Comparison between ultrasound and magnetic resonance imaging in assessment of fetal cytomegalovirus infection. Prenat Diagn 2008;28:753-8.


52. Leruez-Ville M, Stirnemann J, Sellier Y, Guilleminot T, Dejean A, Magny JF, et al. Feasibility of predicting the outcome of fetal infection with cytomegalovirus at the time of prenatal diagnosis. Am J Obstet Gynecol 2016;215:342.e1-9.


53. Walker SP, Palma-Dias R, Wood EM, Shekleton P, Giles ML. Cytomegalovirus in pregnancy: to screen or not to screen. BMC Pregnancy Childbirth 2013;13:96.




54. Picone O, Vauloup-Fellous C, Cordier AG, Parent Du Châtelet I, Senat MV, Frydman R, et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 2009;116:818-23.


55. Vauloup-Fellous C, Picone O, Cordier AG, Parent-du-Châtelet I, Senat MV, Frydman R, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol 2009;46(Suppl 4):S49-53.

56. Reichman O, Miskin I, Sharoni L, Eldar-Geva T, Goldberg D, Tsafrir A, et al. Preconception screening for cytomegalovirus: an effective preventive approach. BioMed Res Int 2014;2014:135416.




57. Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clin Infect Dis 2013;56:1428-35.


58. Grangeot-Keros L, Mayaux MJ, Lebon P, Freymuth F, Eugene G, Stricker R, et al. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J Infect Dis 1997;175:944-6.


59. Ely JW, Yankowitz J, Bowdler NC. Evaluation of pregnant women exposed to respiratory viruses. Am Fam Physician 2000;61:3065-74.

60. Guerra B, Simonazzi G, Puccetti C, Lanari M, Farina A, Lazzarotto T, et al. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 2008;198:380.e1-7.


61. Picone O, Teissier N, Cordier AG, Vauloup-Fellous C, Adle-Biassette H, Martinovic J, et al. Detailed in utero ultrasound description of 30 cases of congenital cytomegalovirus infection. Prenat Diagn 2014;34:518-24.


62. Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011;17:1285-93.


63. Enders G, Bäder U, Lindemann L, Schalasta G, Daiminger A. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat Diagn 2001;21:362-77.


64. Liesnard C, Donner C, Brancart F, Gosselin F, Delforge ML, Rodesch F. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstet Gynecol 2000;95(6 Pt 1):881-8.


65. Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, et al. Congenital cytomegalovirus: a European expert consensus statement on diagnosis and management. Pediatr Infect Dis J 2017;36:1205-13.

66. Leruez-Ville M, Magny JF, Couderc S, Pichon C, Parodi M, Bussières L, et al. Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis 2017;65:398-404.

67. Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 2017;17:e177-88.


68. Hertel L. Human cytomegalovirus tropism for mucosal myeloid dendritic cells. Rev Med Virol 2014;24:379-95.




69. Blanco-Lobo P, Bulnes-Ramos Á, McConnell MJ, Navarro D, Pérez-Romero P. Applying lessons learned from cytomegalovirus infection in transplant patients to vaccine design. Drug Discov Today 2016;21:674-81.


70. Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 2010;84:1005-13.



71. Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol 2008;89(Pt 4):853-65.


72. Fu TM, An Z, Wang D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 2014;32:2525-33.


74. Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 2004;78:10023-33.




75. Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 2005;102:18153-8.



76. Barber V, Calvert A, Vandrevala T, Star C, Khalil A, Griffiths P, et al. Prevention of acquisition of cytomegalovirus infection in pregnancy through hygiene-based behavioral interventions: a systematic review and gap analysis. Pediatr Infect Dis J 2020;39:949-54.


77. Jacquemard F, Yamamoto M, Costa JM, Romand S, Jaqz-Aigrain E, Dejean A, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 2007;114:1113-21.


78. Plotogea M, Isam AJ, Frincu F, Zgura A, Bacinschi X, Sandru F, et al. Overview of cytomegalovirus infection in pregnancy. Diagnostics (Basel) 2022;12:2429.


79. Shahar-Nissan K, Pardo J, Peled O, Krause I, Bilavsky E, Wiznitzer A, et al. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet 2020;396:779-85.


80. Faure-Bardon V, Fourgeaud J, Stirnemann J, Leruez-Ville M, Ville Y. Secondary prevention of congenital cytomegalovirus infection with valacyclovir following maternal primary infection in early pregnancy. Ultrasound Obstet Gynecol 2021;58:576-81.



81. Egloff C, Sibiude J, Vauloup-Fellous C, Benachi A, Bouthry E, Biquard F, et al. New data on efficacy of valacyclovir in secondary prevention of maternal-fetal transmission of cytomegalovirus. Ultrasound Obstet Gynecol 2023;61:59-66.



82. De la Calle M, Baquero-Artigao F, Rodríguez-Molino P, Cabanes M, Cabrera M, Antolin E, et al. Combined treatment with immunoglobulin and valaciclovir in pregnant women with cytomegalovirus infection and high risk of symptomatic fetal disease. J Matern Fetal Neonatal Med 2022;35:3196-200.


83. Zammarchi L, Lazzarotto T, Andreoni M, Campolmi I, Pasquini L, Di Tommaso M, et al. Management of cytomegalovirus infection in pregnancy: is it time for valacyclovir? Clin Microbiol Infect 2020;26:1151-4.


84. Codaccioni C, Vauloup-Fellous C, Letamendia E, Saada J, Benachi A, Vivanti AJ. Case report on early treatment with valaciclovir after maternal primary cytomegalovirus infection. J Gynecol Obstet Hum Reprod 2019;48:287-9.


85. Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005;353:1350-62.


86. Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014;370:1316-26.


87. Kagan KO, Enders M, Schampera MS, Baeumel E, Hoopmann M, Geipel A, et al. Prevention of maternal-fetal transmission of cytomegalovirus after primary maternal infection in the first trimester by biweekly hyperimmunoglobulin administration. Ultrasound Obstet Gynecol 2019;53:383-9.



88. Hughes BL, Clifton RG, Rouse DJ, Saade GR, Dinsmoor MJ, Reddy UM, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med 2021;385:436-44.



89. Kagan KO, Enders M, Hoopmann M, Geipel A, Simonini C, Berg C, et al. Outcome of pregnancies with recent primary cytomegalovirus infection in first trimester treated with hyperimmunoglobulin: observational study. Ultrasound Obstet Gynecol 2021;57:560-7.



90. Hamilton ST, Marschall M, Rawlinson WD. Investigational antiviral therapy models for the prevention and treatment of congenital cytomegalovirus infection during pregnancy. Antimicrob Agents Chemother 2020;65:e01627-20.




91. Rouse DJ, Fette LM, Hughes BL, Saade GR, Dinsmoor MJ, Reddy UM, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol 2022;139:400-6.



- TOOLS
-
METRICS

- Related articles in Obstet Gynecol Sci
-
Changes in pulmonary function during normal pregnancy.1991 March;34(3)
Transabdominal chorionic villus sampling in continuing pregnancies.1991 June;34(6)
A study for acute pyelonephritis during pregnancy.1992 June;35(6)
A case of myasthenia gravis in pregnancy.1992 June;35(6)
Shake test in preterm and term pregnancy.1992 November;35(11)



















