|
 |
- Search
| Obstet Gynecol Sci > Volume 67(1); 2024 > Article |
|
Abstract
Objective
Uterine leiomyoma is a common gynecological condition that negatively affects womenŌĆÖs quality of life. Vitamin D plays an important role in tumor development and progression. However, clinical studies comparing serum vitamin D levels between women with and without uterine leiomyomas are limited and inconclusive. This study aimed to compare serum vitamin D levels in women with and without uterine leiomyomas.
Methods
This hospital-based case-control study included 150 women who visited a gynecological clinic. The cases included 75 women with uterine leiomyoma, whereas the controls included 75 age-and parity-matched participants without uterine leiomyoma. Serum vitamin D levels were measured in each participant and volumes of the uterine leiomyomas were determined using the water displacement method following myomectomy. The statistical significance was inferred at P<0.05.
Results
The mean serum vitamin D level was 15.26┬▒4.96 ng/mL and 22.45┬▒6.93 ng/mL for the case and control groups, respectively. The difference was statistically significant (t-value ŌłÆ7.302 and P<0.001). Within the fibroid group, nine (12.0%), 49 (65.33%), and 17 (22.67%) participants had vitamin D deficiency, insufficiency, and sufficiency, respectively; and in the control group, two (2.67%), 24 (45.33%), and 39 (52.0%) participants had vitamin D deficiency, insufficiency, and sufficiency, respectively. There was significant negative correlation between the fibroid volume and the serum vitamin D level (r=ŌłÆ0.591, P<0.001).
Uterine leiomyoma, also called uterine myoma or uterine fibroids, is a benign tumor arising from the smooth muscles of the uterus [1,2]. They are primarily composed of smooth muscles and varying amounts of connective tissues that contain collagen, fibronectin, and proteoglycans. They are surrounded by a pseudo-capsule of compressed muscle fibers [3] with each arising from neoplastic transformation of a single smooth muscle cell [4]. Uterine leiomyomas are the commonest gynecological tumor worldwide [4-6] and are present in about 70.0% to 80.0% of uteri removed at hysterectomy [7,8]. The cumulative incidence of uterine leiomyoma increases with age and is approximately 40.0-60.0% by the age of 35 years and approximately 70.0-80.0% by the age of 50 years [4,9].
Uterine leiomyoma is usually asymptomatic [4,10,11], however it can present with symptoms such as menorrhagia, abdominal mass and distension, pelvic pain, dysmenorrhea, dyspareunia, non-puerperal uterine inversion, and pressure symptoms [4,9,12-14]. In pregnancy, it can lead to miscarriage, intrauterine growth restriction, preterm labor, obstructed labor, increased incidence of caesarean section, and postpartum hemorrhage [7]. Hence, uterine leiomyomas negatively impact the quality of life and reproductive health of women [11].
Several risk factors are implicated in the development of uterine leiomyoma, including age and the presence of hormones such as estrogen and progesterone [12,15]. For instance, uterine leiomyomas rarely appear before menarche but regress after menopause [1,4,16] and women with early menarche have an increased risk of uterine leiomyoma [4]. These observations suggest that estrogen and progesterone play a role in tumor proliferation [2,12,15,16]. Other risk factors that have been noted include African descent, obesity, alcohol consumption, and caffeine intake [4,12].
Treatment of uterine leiomyomas depends on the patientŌĆÖs presentation and reproductive status. Options include hysterectomy, myomectomy, minimally invasive procedures (like uterine artery embolization, magnetic resonance-guided focused ultrasound surgery, ultrasound guided ablation) and medical treatment (using gonadotropin releasing hormone analogues, antifibrinolytic agents, combined oral contraceptives, non-steroidal anti-inflammatory drugs, etc.) [4,6,17]. Currently, there is no effective medical treatment for uterine leiomyoma [10,17]. Medical treatment is only used to control symptoms and for short-term therapy because of the significant risks associated with long-term therapy, or lack of evidence regarding the benefits and risks of long-term therapy with the newer medical agents [4]. Surgery is the most effective treatment for uterine leiomyomas. However, there is a strong aversion to surgery owing to the attendant reproductive health implications, financial costs, and complications. Considering the enormous financial burden of this common disease on the health care system, a safe, effective and inexpensive medical treatment for uterine leiomyoma would be a major advance in the field and would have immense impact on womenŌĆÖs health worldwide [17]. One such newer agent that has been suggested is vitamin D [4]. Authors have documented that uterine leiomyoma is more prevalent among women with vitamin D deficiency [3,13,17,18] and others have reported that vitamin D could prevent an increase in the volume of uterine fibroids [19] or even shrink them [20]. It has been suggested that vitamin D deficiency correlates with the risk and burden of uterine leiomyoma [4]. Any association between uterine leiomyoma and vitamin D could be exploited in prevention or treatment.
Vitamin D is a fat-soluble vitamin. Endogenously, vitamin D is produced as vitamin D3 (cholecalciferol), whereas dietary vitamin D is produced as vitamin D2 (ergocalciferol) or D3 (cholecalciferol). Uterine leiomyoma and low vitamin D levels are more prevalent in black than Caucasian women [21,22]. Vitamin D deficiency is estimated to be 10 times more common in African American women (40.0%) than in Caucasian women (4.0%) and could partially explain the differences in pathogenesis and clinical manifestations of uterine leiomyoma between them [23].
Various mechanisms have been suggested to explain the inhibition of uterine fibroid growth by vitamin D. Vitamin D inhibits growth of uterine fibroid cells through the down-regulation of proliferating cell nuclear antigen, the cell cycle regulatory protein cyclin-dependent kinase (CDK) 1, and antiapoptotic proteins B-cell lymphoma 2 (BCL-2) and BCL-w, as well as, through the suppression of catechol-O-methyltransferase (COMT) expression and activity in the uterine fibroid cells [24]. CDK1 is required for G2-M transition during the cell cycle and vitamin D treatment reduced CDK1 expression in uterine fibroid cells. Vitamin D reduced COMT messenger ribonucleic acid and protein expression, inhibited COMT enzyme activity in uterine fibroid cells [24] and significantly reduced the protein expression of matrix metalloproteinase-2 and ŌłÆ9 in uterine fibroid cells compared to controls. Uterine fibroid cells are rich in MMP and have been implicated in fibroid pathogenesis [25].
Despite the promise of vitamin D as a potential medical agent for the prevention and treatment of uterine leiomyomas, studies comparing serum vitamin D levels in populations with and without uterine leiomyomas are scarce. Most studies have been conducted in Caucasian women, where the prevalence of uterine leiomyoma is low. This case-control study aimed to compare serum vitamin D levels between women with and without uterine leiomyomas.
This was a hospital-based, case-controlled clinical study. Study participants were recruited from among consenting premenopausal women aged 25-45 years who presented to the gynecological clinic and met the inclusion criteria. The patients were women with an ultrasound diagnosis of uterine leiomyomas with or without clinical manifestations. The controls were recruited from participants visiting the gynecology clinic for conditions other than uterine leiomyomas as well as apparently healthy volunteers (including hospital staff) who were of the same age as the cases ┬▒2 years and were also matched for parity ┬▒2. Controls underwent ultrasonography to rule out uterine fibroids. Written informed consent was obtained from eligible candidates before participation in the study. Recruitment continued until the sample size was reached which took 8 months (from October 2020 to May 2021). Women with one or more of the following conditions in their medical history were excluded: prior myomectomy or hysterectomy; suspected adenomyosis; menopause; malignancy; hypertension; diabetes; autoimmune disorders; and coronary, hepatic, or renal diseases. Other exclusion criteria included current or past history of the following within the last 6 months: pregnancy, primary amenorrhea, lactation, abortion/miscarriage, vitamin D supplementation, and hormonal treatment (including oral contraceptives). Ethical approval was obtained before the commencement of the study.
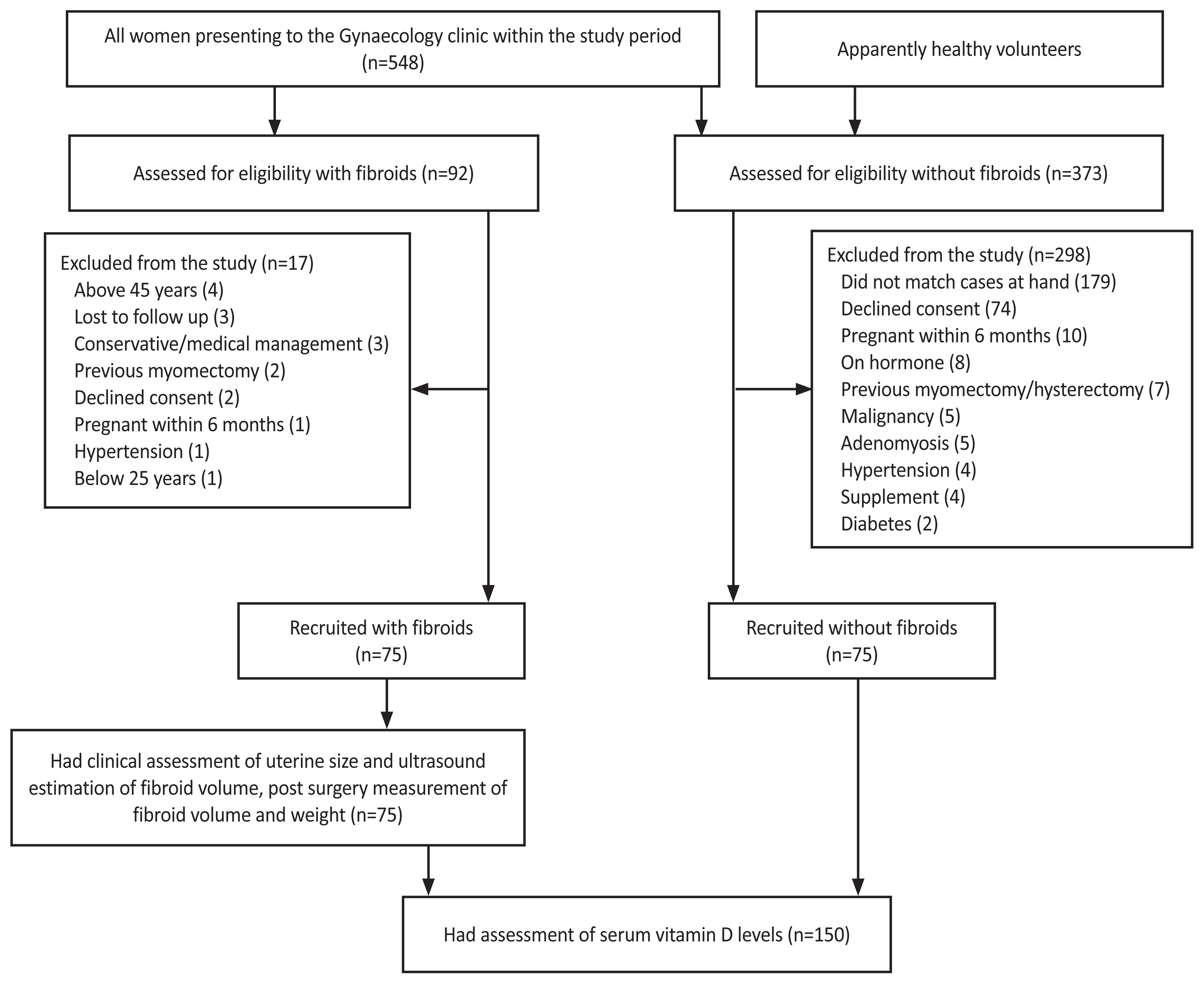
We estimated that a sample size of 120 with a 1:1 case to control ratio (60 cases and 60 controls) would allow us to accept a two-tailed alpha error of 0.05 with 90.0% power using a mean of 24.45 ng/mL and standard deviation of 8.13 ng/mL for cases and mean of 29.53 ng/mL for controls as reported in a previous study by Sabry et al. [17] However, we recruited 75 consenting participants in the case and control groups respectively to account for possible loss of sample and withdrawal of consent. Within the 8 months of the study, a total of 548 women were seen at the gynecology clinic out of which, 92 (16.8%) had a diagnosis of uterine fibroids but only 75 were recruited for the study (as cases). Seventy-five matched participants without uterine fibroids were recruited as controls. Fig. 1 shows a recruitment flowchart.
Demographic details, brief medical history, and findings on physical examination, including biometric measurements and bimanual examination, were collected using Proformas. Venous blood samples were collected, allowed to clot, and centrifuged at 2,500 rpm for 10 minutes. The serum was separated and frozen at -20°C until analysis using a commercially available kit produced by the Calbiotech®, Inc. (Spring Valley, CA, USA). This kit is a solid-phase enzyme-linked immunoassay and is based on competitive binding [26]. The serum vitamin D levels are expressed in ng/mL.
According to the World Health Organization recommendations, women were grouped into three different groups with respect to vitamin D status: 25-hydroxyvitamin D3 deficiency with levels <10 ng/mL, insufficiency with levels between 10-19.9 ng/mL, and sufficiency with levels of Ōēź20 ng/mL [27,28]. No unanimity exists as to the ideal vitamin D levels for optimal health. Nonetheless, the Institute of Medicine (IOM) recommends a level of 20 ng/mL as optimal [29].
The control participants underwent transvaginal ultrasonography unless transabdominal ultrasonography was performed for participants with an intact hymen or other contraindications for transvaginal scanning. For the case participants, following myomectomy, the volume of the uterine leiomyoma was determined by the water displacement method using a measuring cylinder [30]. This is based on ArchimedesŌĆÖ principle, which states that when an object is totally immersed in a fluid, the volume of the fluid displaced is equal to the volume of the object [30]. The increase in the volume of water in the measuring cylinder is equal to the volume of the uterine leiomyoma, expressed in milliliters (mL). The weight of the fibroid mass (g) was measured using a scale and the diagnosis of uterine fibroids was confirmed by histological examination of the specimens.
The analysis was performed using Statistical Package for Social Sciences (SPSS) version 23 (IBM Corporation, Armonk, NY, USA). Continuous data were presented as means and standard deviations. Categorical variables were analyzed using chi-square tests where appropriate, whereas continuous data were analyzed using t-tests. Statistical significance was inferred at P-value Ōēż0.05.
The age of the participants ranged from 25 to 45 years with a mean age of 35.25┬▒4.91 and 25 to 44 years with a mean age of 35.34┬▒4.88 for the case and control groups, respectively. The other sociodemographic characteristics of the participants are shown in Table 1. The total post-surgery fibroids volume ranged from 85 mL to 3,695 mL with a mean volume of 1,394.25┬▒996.57 mL. Similarly, the total weight ranged from 90 g to 4,254 g with a mean of 1,469┬▒1,060.48 g.
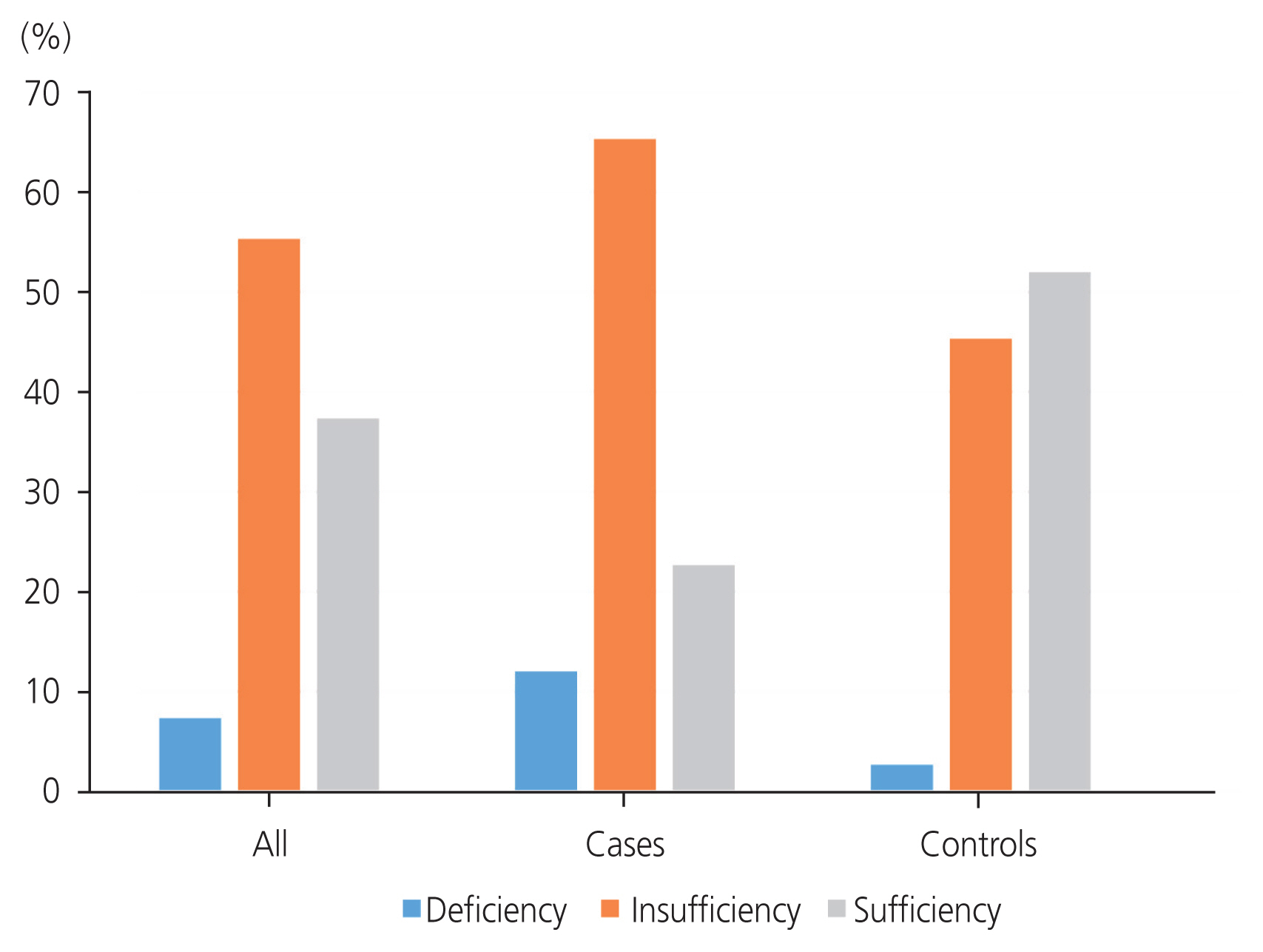
Overall, the serum vitamin D levels of all the participants ranged from 7.7 ng/mL to 35.4 ng/mL with mean serum vitamin D levels of 18.86┬▒7.01 ng/mL (Table 2). Assessment of overall vitamin D status showed that 11 (7.33%), 83 (55.33%), and 56 (37.33%) participants had vitamin D deficiency, insufficiency, and sufficiency, respectively. For the fibroids group, the serum vitamin D levels ranged from 7.7 ng/mL to 29.3 ng/mL and, for the control group, ranged from 9.3 ng/mL to 35.4 ng/mL. The mean serum vitamin D level was 15.26┬▒4.96 ng/mL and 22.45┬▒6.93 ng/mL for the case and control groups, respectively. The difference in the mean serum vitamin D levels was statistically significant (t-value ŌłÆ7.302 and P-value <0.001). Within the fibroid group, 9 (12.0%), 49 (65.33%), and 17 (22.67%) women had vitamin D deficiency, insufficiency, and sufficiency, respectively (Fig. 2 and Table 3). Within the control group, two (2.67%), 24 (45.33%), and 39 (52.0%) women had vitamin D deficiency, insufficiency, and sufficiency, respectively (Fig. 2 and Table 3). Thus, 49 participants (65.33%) in the fibroid group had vitamin D insufficiency, whereas 39 participants (52.0%) in the control group had vitamin D sufficiency. Table 3 shows that the relationship between vitamin D status in the case and control participants was statistically significant (Žć2-value 15.808; P-value <0.001). The overall prevalence of hypovitaminosis D in this study was 7.33%: 12.00% in the fibroid group and 2.67% in the control group (Fig. 2). The difference in prevalence between the case and control groups was statistically significant (P<0.001) (Table 3).
After checking for the normal distribution of serum vitamin D levels among the different sizes of uterine fibroids and found to be normally distributed, a parametric one-way analysis of variance was performed to test for significance (Table 4). The mean difference between the groups was statistically significant (P<0.001) and parametric Pearson correlation analysis was performed to establish linear relationships among the variables. Table 5 shows the magnitude of the correlation between the variables studied within the case group. Statistical significance was set at P<0.05. There was a significantly strong negative correlation between the weight of the fibroid masses and serum vitamin D level (r=ŌłÆ0.601; P<0.001). Similarly, there was significant strong negative correlation between the fibroid volume and the level of serum vitamin D (r=ŌłÆ0.591; P<0.001)
The mean serum vitamin D levels of 18.86 ng/mL of all the participants reported in this study is higher than the 9.05 ng/mL reported by Ingala et al. [12] in the Democratic Republic of Congo (DRC), 10.4 ng/mL among black women in the United States and 14.6 ng/mL among a mixed population in the United States [13]. It is, however, less than the 20.7 ng/mL reported in a white population by Baird et al. [13]. The overall vitamin D deficiency of 7.33% in our study is comparable to 13.7% as reported in a previous study by Ingala et al. [12]. Similarly, a vitamin D insufficiency of 55.33% that we reported is comparable to 48.4% previously reported by Ingala et al. [12]. Only 37.33% of the participants have sufficient vitamin D status. This proportion of participants with vitamin D sufficiency status, however, differs from the findings of a previous study among a mixed race in the United States by Baird et al. [13] and among black people in the DRC by Ingala et al. [12] which reported that only 26.0% and 29.9% of the participants, respectively, had sufficient vitamin D levels. These findings are consistent with previous studies that reported that black people have low vitamin D levels [22,31,32]. Even though the participants live in the tropics where there is a high amount of sunlight, high melanin concentration in the skin of black people decreases the absorption of ultraviolet rays from the sun, thus predisposing them to vitamin D deficiency [10,33] and there is a decreased dietary intake of vitamin D among Africans [10,33].
The mean serum vitamin D level among the cases of 15.26┬▒4.96 ng/mL is comparable to 18.0 ng/mL reported among cases in Italy by Paffoni et al. [18], 19.7 ng/mL reported among a mixed population in Egypt by Sabry et al. [17], and 12.9 ng/mL reported in a black population by the same study in Egypt. It is, however, lower than the 24.45 ng/mL reported among a white population in Egypt by Sabry et al. [17]. Similarly, the mean serum vitamin D level among the controls in this study (22.45┬▒6.93 ng/mL) is comparable to that from previous studies by Sabry et al. [17] and Paffoni et al. [18], with levels of 20.8 ng/mL and 22.3 ng/mL respectively. It is, however, greater than 18.30 ng/mL among black people in Egypt but less than 29.53 ng/mL among whites women without uterine fibroids [17]. The difference in the mean serum vitamin D levels between the cases and controls in this study was statistically significant (t-value ŌłÆ7.302 and P-value <0.001). This is in agreement with studies from Italy [18], United States of America [13] and Egypt [17] which reported lower serum vitamin D levels in women with uterine fibroids. The subgroup analysis in the Egyptian study reported lower serum vitamin D levels among cases in both black and white populations. This finding differs from that of a previous work [12] which reported no significant difference in the mean serum vitamin D levels between cases and controls.
Most, 49 participants (65.33%), in the fibroid group had vitamin D insufficiency while most, 39 (52.0%), of the participants in the control group had vitamin D sufficiency. This difference in vitamin D status is statistically significant (Žć2-value 15.808; P-value <0.001). Compared to the controls (2.67%), there were more women with vitamin D deficiency (12.0%). Conversely, there were fewer women with vitamin D sufficiency among the cases (22.67%) than among the controls (52.0%). This agrees with findings from previous studies that reported a higher prevalence of vitamin D deficiency among cases than in controls [12,18].
A possible reasons for the varied prevalence of vitamin D deficiency among these studies include differences in nutritional habits, exposure to sunlight, mode of dressing, skin color, seasonal variations, body mass of the participants and their locality [34]. Vitamin D deficiency has a very wide prevalence that ranges from 18.0% to 84.0% depending on the place of residence, ethnicity, local clothing customs and dietary intake as well as the cut off used [35]. Again, while some authors regard vitamin D levels less than 20 ng/mL as deficient, others put the cut off at less than 10 ng/mL [13,18]. For example, in the study by Ingala et al. [12], vitamin D deficiency was defined as serum vitamin D levels less than 4 ng/mL in one arm (using local criteria) and less than 12 ng/mL in another arm (using IOM criteria) while deficiency was defined as serum vitamin D levels less than 10 ng/mL in the study done by Paffoni et al. [18].
Consistent with the study by Paffoni et al. [18] in Italy, this study found a strong negative correlation between fibroid volume, measured using the water displacement method, and serum vitamin D levels. The strong significant negative correlation between the weight of the fibroid masses and the level of serum vitamin D reported in this study implies that for participants with uterine fibroids, the lower the serum vitamin D level, the higher the weight of the fibroids, and vice versa. This observation gives credence to the thought that vitamin D inhibits the growth of uterine fibroids to large sizes as reported by Sharan et al. [24] and Halder et al. [36]. This inhibition is higher at higher vitamin D levels [24]. This observation is also similar to the findings by Bl├żuer et al. [37] in Finland. This can partly explain why black people who have reduced vitamin D levels also have larger fibroid masses [21].
One of the strengths of this study was the elimination bias through appropriate matching of participants in the two groups. Women with uterine fibroids were matched appropriately by age and parity with the controls. There were no significant differences in the mean age or parity of the women. The participants were recruited by strictly complying with the inclusion and exclusion criteria, thereby protecting the results from confounders. The chemical pathologist who analyzed the samples was blinded to the group to which each sample belonged.
In this study, women were screened for fibroids with an ultrasound scan, unlike in other studies where recruitment into cases or controls was based on self-reporting of the presence or absence of fibroids. Several patients with uterine fibroids are asymptomatic. Again, unlike other similar studies where the size of the uterine leiomyoma was determined by ultrasound scan estimation, in this study, the size of the leiomyoma was determined using the liquid displacement method, which has been described as the gold standard [38]. Large inter-observer variability is a recognized limitation of volume estimation by ultrasound scan using the ellipsoid formula [38]. Again, it is not uncommon for ultrasound to erroneously diagnose other uterine masses as leiomyomas. In addition to ultrasound diagnosis, efforts were made in this study to ensure that the masses identified as uterine leiomyomas had intraoperative features consistent with leiomyomas, which were confirmed by histological reports.
A limitation of this study was its cross-sectional design. Serum vitamin D levels were not assessed in the cases prior to leiomyoma onset. Serum vitamin D levels at the time of recruitment may not directly reflect the vitamin D status during the years preceding recruitment when leiomyomas developed. Another limitation is that this was a single-center study with a small sample size.
This study showed that women with uterine leiomyomas had significantly lower vitamin D levels than those without uterine leiomyomas. Even among women with leiomyomas, the larger the fibroid mass, the lower were the vitamin D levels. These findings highlight the role of vitamin D in the etiology and pathogenesis of uterine leiomyomas. Our findings suggest that vitamin D deficiency favors the development and growth of uterine fibroids. It does not focus on the contribution of other known risks to the development and progression of uterine fibroids.
However, further research, possibly in a multicenter setting, is warranted to demonstrate this causal relationship. It is also important to determine whether vitamin D deficiency favors the development and/or growth of leiomyomas. This finding may have a profound impact on the potential prophylactic and/or therapeutic use of vitamin D supplementation for uterine leiomyomas. Therefore, vitamin D supplementation may be a simple and economical means of preventing the development or growth of fibroids. The present study serves as an impetus and foundation for a more robust multicenter study in other regions. This will be of immense benefit in elucidating the role of vitamin D in the pathogenesis of uterine leiomyomas.
Notes
Ethical approval
Approval was obtained from Nnamdi Azikiwe University Teaching Hospital Ethics Committee (Approval number: NAUTH/CS/66/VOL.10/204/2017/118). All subjects recruited for this study gave consent to participate.
Table┬Ā1
The socio-demographic characteristics of the subjects
Table┬Ā2
Student t-test showing the age differences, parity and mean levels of serum vitamin D among all subjects, case subjects and control subjects (P<0.05)
| Variable | All (n=150) | Case group (n=75) | Control group (n=75) | t-value | P-value |
|---|---|---|---|---|---|
| Mean age (yr) | 35.3┬▒4.88 | 35.25┬▒4.91 | 35.34┬▒4.88 | ŌłÆ0.116 | 0.907 |
| Mean parity | 0.70┬▒1.05 | 0.69┬▒1.05 | 0.72┬▒1.05 | ŌłÆ0.154 | 0.877 |
| Serum vitamin D (ng/mL) | 18.86┬▒7.01 | 15.26┬▒4.96 | 22.45┬▒6.93 | ŌłÆ7.302 | <0.001a) |
Table┬Ā3
Chi-square analysis showing the comparison between vitamin D status in case and control subjects (P<0.05)
| Laboratory finding | Case group (n=75) | Control group (n=75) | Žć2-value | P-value |
|---|---|---|---|---|
| Serum vitamin D status | ||||
| ŌĆāDeficiency, less than 10 ng/mL | 9 (12.0) | 2 (2.67) | ||
| ŌĆāInsufficiency, 10-19.9 ng/mL | 49 (65.33) | 24 (45.33) | 15.808 | <0.001a) |
| ŌĆāSufficiency, greater than 20 ng/mL | 17 (22.67) | 39 (52.0) | ||
| Total | 75 (100.0) | 75 (100.0) | ||
Table┬Ā4
One-way ANOVA showing serum vitamin D levels (ng/mL) among subjects with different sizes (volume and weight) of uterine fibroids within the cases (P<0.05)
| N | Mean┬▒SD | F-value | P-value | |
|---|---|---|---|---|
| Volume of fibroid (mL) | ||||
| ŌĆā0-1,000 mL (A) | 30 (40.0) | 18.20┬▒5.20 | ||
| ŌĆā1,001-2,000 mL (B) | 30 (40.0) | 14.22┬▒3.97 | 10.35 | <0.001a) |
| ŌĆā2,001-3,000 mL (C) | 5 (6.67) | 14.02┬▒1.14 | ||
| ŌĆā3,001-4,000 mL (D) | 10 (13.33) | 10.21┬▒1.42 | ||
| Weight of fibroids (g) | ||||
| ŌĆā0-1,000 g (E) | 24 (32.0) | 20.21┬▒3.70 | ||
| ŌĆā1,001-2,000 g (F) | 36 (48.0) | 14.05┬▒1.31 | ||
| ŌĆā2,001-3,000 g (G) | 4 (5.3) | 13.54┬▒3.87 | 18.81 | <0.001a) |
| ŌĆā3,001-4,000 g (H) | 8 (10.7) | 10.57┬▒1.72 | ||
| ŌĆā4,001-5,000 g (I) | 3 (4.0) | 10.46┬▒2.21 |
Table┬Ā5
Pearson correlation showing the levels of linear association between variables within the case subjects (P<0.05)
| Variable | Coefficient of correlation (r) | P-value |
|---|---|---|
| Age (yr) and vitamin D level (ng/mL) | 0.449 | <0.001a) |
| Fibroid weight (g) and vitamin D level (ng/mL) | ŌłÆ0.601 | <0.001a) |
| Fibroid volume (mL) and vitamin D level (ng/mL) | ŌłÆ0.591 | <0.001a) |
| Fibroid volume (mL) and duration of symptoms (yr) | 0.113 | 0.333 |
| Uterine size (weeks) and duration of symptoms (yr) | 0.162 | 0.164 |
| Uterine size (weeks) and vitamin D level (ng/mL) | ŌłÆ0.622 | <0.001a) |
| Number of fibroids and vitamin D level (ng/mL) | ŌłÆ0.349 | 0.002a) |
References
1. Segars JH, Parr ott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update 2014;20:309-33.



2. Ukwenya V, Maduemezia N, Afolayan O, Alese O, Thomas W. Prevalence of uterine fibroid in a South-Western Nigerian population: a sonographic study. J Exp Clin Anat 2015;14:24-9.

3. Al-hendy A, Badr M. Can vitamin D reduce the risk of uterine fibroids? Womens Health (Lond) 2014;10:353-8.




4. Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health 2014;6:95-114.



5. Ezeama C, Ikechebelu J, Obiechina Nj, Ezeama N. Clinical presentation of uterine fibroids in Nnewi, Nigeria: a 5-year review. Ann Med Health Sci Res 2012;2:114-8.



6. Okogbo FO, Ezechi OC, Loto OM, Ezeobi PM. Uterine leiomyomata in South Western Nigeria: a clinical study of presentations and management outcome. Afr Health Sci 2011;11:271-8.


7. McCool WF, Durain D, Davis M. Overview of latest evidence on uterine fibroids. Nurs Womens Health 2014 18:314-31. quiz 332.


8. Fasubaa OB, Sowemimo OO, Ayegbusi OE, Abdur-Rahim ZF, Idowu BS, Ayobami O, et al. Contributions of uterine fibroids to infertility at Ile-Ife, South-Western Nigeria. Trop J Obs Gynaecol 2018;35:266-70.

9. Catherino WH, Eltoukhi HM, Al-hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med 2013;31:370-9.



10. Brakta S, Diamond JS, Al-hendy A, Diamond MP, Halder SK. Role of vitamin D in uterine fibroid biology. Fertil Steril 2015;104:698-706.



11. Ekine AA, Lawani LO, Iyoke CA, Jeremiah I, Ibrahim IA. Review of the clinical presentation of uterine fibroid and the effect of therapeutic intervention on fertility. Am J Clin Med Res 2015;3:9-13.
12. Ingala P, Mboloko J, Tshiband A, Lepira F, Kayembe P, Lebwaze B, et al. Vitamin D deficiency and risk of uterine leiomyoma among congolese women. A hospital-based case-control study. Am Sci Res J Eng Technol Sci 2016;22:126-37.
13. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology 2013;24:447-53.



14. Umeononihu OS, Adinma JI, Obiechina NJ, Eleje GU, Udegbunam OI, Mbachu II. Uterine leiomyoma associated non-puerperal uterine inversion misdiagnosed as advanced cervical cancer: a case report. Int J Surg Case Rep 2013;4:1000-3.



15. Ciavattini A, Di Giuseppe J, Stortoni P, Montik N, Giannubilo SR, Litta P, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int 2013;2013:173184.




16. Garba I, Ayyuba R, Adewale TM, Abubakar IS. Surgical management of uterine fibroids at Aminu Kano Teaching Hospital. Niger J Basic Clin Sci 2016;13:50-4.

17. Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health 2013;5:93-100.


18. Paffoni A, Somigliana E, ViganoŌĆÖ P, Benaglia L, Cardellicchio L, Pagliardini L, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab 2013;98:E1374-8.


19. Arjeh S, Darsareh F, Asl ZA, Azizi Kutenaei M. Effect of oral consumption of vitamin D on uterine fibroids: a randomized clinical trial. Complement Ther Clin Pract 2020;39:101159.


20. Hajhashemi M, Ansari M, Haghollahi F, Eslami B. The effect of vitamin D supplementation on the size of uterine leiomyoma in women with vitamin D deficiency. Caspian J Intern Med 2019;10:125-31.


21. Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967-73.


22. Okoro CC, Udigwe GO, Eleje GU, Ikpeze OC, Enechukwu CI, Egeonu RO, et al. Association between serum hypovitaminosis D and preeclampsia: a nested case-control study. Magna Scientia Adv Res Rev 2023;07:009-17.
23. Halder SK, Goodwin JS, Al-Hendy A. 1,25-dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab 2011;96:E754-62.


24. Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril 2011;95:247-53.



25. Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and ŌłÆ9 in human uterine fibroid cells. Hum Reprod 2013;28:2407-16.



26. Calbiotech. 25(OH) vitamin D ELISA package insert [Internet] El Cajon: Calbiotech; c2014 [cited 2023 Apr 30]. Available from: https://cdn.shopify.com/s/files/1/0538/1921/1960/files/VD220B_RC_R19.pdf?v=1615691805
.
27. World Health Organization. Vitamin D and cancer [Internet] Lyon Cedex: World Health Organization; c2008 [cited 2023 Apr 30]. Available from: https://publications.iarc.fr/_publications/media/download/4047/e8395c0b97f8bfa9bd82e019e5379df1a9345355.pdf
.
29. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011.
30. Karges JR, Mark BE, Stikeleather SJ, Worrell TW. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther 2003;83:134-45.



31. Mitro SD, Zota AR. Vitamin D and uterine leiomyoma among a sample of US women: findings from NHANES, 2001-2006. Reprod Toxicol 2015;57:81-6.



32. Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin--a comparison of black and Caucasian skin. J Am Acad Dermatol 1979;1:249-60.


34. Das G, Crocombe S, McGrath M, Berry JL, Mughal MZ. Hypovitaminosis D among healthy adolescent girls attending an inner city school. Arch Dis Child 2006;91:569-72.



35. Behjat Sasan S, Zandvakili F, Soufizadeh N, Baybordi E. The effects of vitamin D supplement on prevention of recurrence of preeclampsia in pregnant women with a history of preeclampsia. Obstet Gynecol Int 2017;2017:8249264.


36. Halder SK, Sharan C, Al-hendy O, Al-hendy A. Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod Sci 2014;21:1108-19.




-
METRICS

-
- 0 Crossref
- Scopus
- 1,326 View
- 199 Download
- Related articles in Obstet Gynecol Sci
-
Dietary pattern and risk of endometrioma in Korean women: a case-control study2021 January;64(1)
Associations between metabolic syndrome and gynecologic cancer2020 May;63(3)
Correlation between the posterior vaginal wall and apex in pelvic organ prolapse2018 July;61(4)