 |
 |
- Search
| Obstet Gynecol Sci > Volume 67(2); 2024 > Article |
|
Abstract
Objective
Controlled ovarian stimulation leads to profound changes in the endocrine characteristics of the ovarian cycle. Serum luteinising hormone (LH) levels on the day of trigger have been shown to correlate with oocyte quality and pregnancy rate in antagonist cycles.
Methods
This is an observational study of 86 women undergoing an antagonist in-vitro fertilisation cycle. Oocyte maturation trigger used was either Inj. human chorionic gonadotropin or Inj. triptorelin 0.2 mg s/c or a combination of both. Women were categorised into four groups based on serum LH levels on the day of trigger i.e., LH ≤0.5 (n=8), LH=0.6-1.0 international units (IU)/L (n=12), LH=1.0-1.5 IU/L (n=13), and LH >1.6 IU/L (n=53) and the subgroup analysis was done based on type of trigger used.
Results
Mature oocyte (MII) retrieval rate did not show a significant relation with serum LH levels (87%, 89%, 77%, and 76% in groups with LH <0.5, 0.5-1.0, 1.0-1.5, and >1.5 IU/L respectively; P-value=0.243). There was no significant difference in the clinical pregnancy rate either when women were split according to the type of trigger given or according to trigger day LH levels. Women with low LH levels (<0.5 IU/L) required significantly more doses of gonadotropins compared to women with LH levels of 1.0-1.5 IU/L. (3,531+1,133 vs. 2,281+938; P-value=0.01).
The aim of an in vitro fertilization (IVF) cycle is to obtain the optimum number of good-quality oocytes and embryos for a healthy baby. Tremendous advancements have been made in IVF since the birth of the first child using this technique. One of the major areas of research is the optimization of the number and quality of retrieved oocytes without compromising the safety of women undergoing IVF [1]. Controlled ovarian stimulation involves an exogenous administration of gonadotropins (follicle stimulating hormone [FSH] and luteinising hormone [LH]) to stimulate the growth of multiple follicles, which leads to changes in the hormonal milieu of the ovaries. The endocrine characteristics of the follicular phase can have a major impact on the quality and quantity of oocytes, and subsequently, on the outcome of an IVF cycle [2]. Recently, there has been tremendous interest in the antagonist protocol owing to its convenience, lower rate of ovarian hyperstimulation, and patient acceptability due to fewer days of injections. However, in antagonist cycles, the pregnancy rates may be lower in fresh embryo transfers [3,4]. Gonadotropin releasing hormone (GnRH) antagonists result in rapid and marked decline in serum LH levels, which might affect the follicle and endometrial development [5]. LH plays a major role in the late follicular development and oocyte maturation during normal ovulatory cycles [6]. Further, serum LH levels on the day of the trigger correlate with the quality of the oocytes and pregnancy rates [7,8]. Optimal LH levels on the day of the trigger are thought to induce optimal corpus luteal function, which may lead to optimal luteal phase steroid hormone levels [9]. Extremes LH levels can have deleterious effects on the quality of recovered oocytes. In animal studies, GnRH antagonist downregulation and LH and serum estradiol (E2) depletion negatively affected embryo development and implantation [10]. In women with hypogonadotropic-hypogonadism, follicular development can be achieved with pure FSH, but the addition of LH or human menopausal gonadotropin (HMG) is required to achieve oocyte maturation and fertilization [11]. Previous studies have highlighted the importance of premature LH increase and its deleterious effect on oocyte quality [12]; subsequently, the impact of very low LH levels in the late follicular phase has also been reported. These studies highlight that LH over suppression can have deleterious effects on oocyte maturation [13]. A few studies have demonstrated that very low LH levels on the day of the trigger can be associated with poor oocyte yield, suboptimal oocyte quality [14,15], and increased chances of pregnancy loss [13], whereas some studies did not demonstrate such an association [16]. Few studies showed that patients with low LH levels on the day of the trigger and who are injected with GnRH agonist as a trigger have a suboptimal oocyte yield; however, this failed to show any significant difference in the pregnancy rate [17]. The current study was conducted to determine whether there was any correlation between serum LH levels on the day of the trigger and IVF outcomes in our population.
This was an observational study of 86 women who underwent antagonist IVF cycles for various indications at a tertiary-level IVF unit in India. Data were extracted from the medical records in a predefined manner by an individual who was not part of the study team. Patient identifiers were removed from the data for anonymization.
The inclusion: criteria were women age group 18-45 years and who underwent antagonist IVF cycles for various indications.
The exclusion criteria were: 1) IVF-cycle cancelled due to any reasons before the day of trigger; 2) LH levels for the day of the trigger or embryological details were not available; 3) deviation from the normal trigger to pick-up time (i.e., 34-36 hours); and 4) women who underwent IVF with hydrosalpinx/pelvic tuberculosis/adenomyosis/severe male factor infertility which could have affected the outcome of the IVF cycle.
Ovarian stimulation was initiated with a subcutaneous injection of recombinant FSH (Inj. Gonal-f; Merck Serono, Darmstadt, Germany) at an appropriate dose on day 2 or day 3. The dose was individualized according to participant baseline parameters such as age, body mass index, ovarian reserve, and previous cycle characteristics. Subsequently, Inj. HMG (Menotropin; Sun Pharmaceuticals, Mumbai, India) was added according to the physician’s discretion, depending on follicular growth. Follicular growth was monitored using serial ultrasonography, and the FSH and HMG doses were adjusted according to the dynamics of follicular growth. GnRH antagonist (Inj. Cetrorelix 0.25 mg s/c; Sun Pharmaceuticals) was initiated when the lead follicle reached a diameter of 12-14 mm and/or the serum estradiol levels were more than 500 pg/mL. Ovarian stimulation was continued until at least three follicles reached a size of 18-20 mm, when the oocyte trigger was planned.
The oocyte maturation trigger used was HCG (urinary 10,000 IU intramuscular (IM) (Inj. Chorionic gonadotropin, Sun Pharma) recombinant human chorionic gonadotropin (rHCG) 250 μg s/c, (Inj. Ovitrelle; Merck Serono), or triptorelin 0.2 mg s/c (Inj. Decapeptyl by Ferring Pharmaceutical, Saint-Prex, Switzerland), or a combination of both, at the discretion of the treating physician. As a departmental protocol, for women with LH <1.5 IU/L, the trigger used was either dual trigger or HCG. None of the patients received only GnRH as a sole trigger. Whether only HCG or a combination of HCG and triptorelin was used as a trigger was noted. Oocyte retrieval was done 35 hours after the HCG trigger using transvaginal sonography under intravenous sedation.
Serum LH, estradiol, and progesterone levels were determined on the day of the final oocyte maturation trigger in all patients. Hormonal evaluation was performed using a fully automated electrochemiluminescence technology (Roche Cobas e411 analyzer; Roche, Mumbai, India). Women were categorized into four groups based on serum LH levels on the day of trigger i.e., LH ≤0.5 IU/L, LH=0.6-1.0 IU/L, LH=1.0-1.5 IU/L, and LH >1.6 IU/L, and a subgroup analysis was done.
Oocytes were assessed using the standard oocyte morphology assessment criteria introduced by Lin et al. [18], and all patients underwent intracytoplasmic sperm Injection. Culture media prepared and produced by Vitrolife (Vitro life, Sweden AB, Gothenburg, Sweden) we used. Fertilization was defined as the presence of two pronuclei at 16-18 hours post insemination/injection. Embryo assessment was done using standard morphological assessment according to Veeck’s modified scoring system [19]. Embryo transfer was done on day 3 or 5 following oocyte retrieval. All patients were advised to take 600 mg micronized progesterone daily as luteal phase support for 2 weeks. The total number of oocytes retrieved, number of mature (mature oocytes [MII]) oocytes retrieved, and MII retrieval rate (obtained by dividing the number of MII/total number of oocytes ×100), and the embryos were graded as grade A or grade B (Veeck’s modified scoring system).
The primary outcomes were the total number of mature (MII) oocytes, the MII retrieval rate (%) (defined as the percentage of MII/total oocytes retrieved), and the number and percentage of grade A embryos (percentage of MII that converted to grade A embryos on day 3).
The secondary outcomes were 1) serum estradiol levels per mature oocyte in these four groups and correlation with the type of trigger used and 2) clinical pregnancy rate (defined as the presence of at least one gestational sac in the uterine cavity 5 weeks after embryo transfer), ongoing pregnancy rate (continuation of viable pregnancy beyond 12 weeks period of gestation), miscarriage rate (loss of clinical pregnancy before 12 weeks of gestation), and live birth rate (defined as delivery of a live infant after 28 gestational weeks) in these groups according to the LH levels.
Data were collected in an anonymized format using Microsoft excel. Continuous variables were expressed as mean and standard deviation, and categorical variables were expressed as percentages. The Kruskal-Wallis test was used to determine the statistical significance between the means across groups for continuous variables. The chi-square test and Fisher’s exact test were used to determine the statistical significance between the groups for categorical variables. Statistical significance was set at P<0.05. SPSS version 16.0 (IBM SPSS, Chicago, IL, USA) was used for all analyses.
The total cohort consisted of 86 women who underwent antagonist cycle IVF due to various indications, and for whom measurements of serum LH levels on the day of the trigger were available (Fig. 1). The trigger administered was either HCG (rHCG 250 μg or urinary HCG 10,000 IU) or decapeptyl 0.2 mg s/c, or a combination of both. Table 1 shows baseline characteristics and ovarian stimulation details. There were no significant differences in the MII oocyte recovery rate, number of oocytes retrieved, or number of grade A embryos retrieved in the different LH groups. All women with LH <1.5 received either a dual trigger or HCG based on the departmental protocol and previous literature findings (Table 2). A decreasing trend gonadotropin requirement was seen from LH levels 0.5 IU/L to 1.5 IU/L, with a difference being statistically significant in the group with LH levels <0.5 and 1.0-1.5 IU/L (Fig. 2). The group with low LH levels required longer days of stimulation although this difference was not statistically significant (Fig. 3).
A higher number of miscarriages were observed in women with LH levels >1.5 IU/L on trigger day i.e., four out of a total of five reported miscarriages were seen in the group with LH levels >1.5 IU/L and one in the group with LH levels=0.5-1.0 IU/L. Another important observation was that a greater number of miscarriages in women i.e., 50% of the reported miscarriages were seen in women with progesterone levels <0.49 when the cases were divided into three groups based on tertiles of progesterone tertile (serum progesterone on day of trigger <0.49, 0.49-0.83, and >0.83 ng/mL).
The MII retrieval rate did not show a significant relationship with serum LH levels (Fig. 4). There were no significant differences in the clinical pregnancy rate when women were divided according to the type of trigger administered or trigger day LH levels (Tables 3, 4).
Similarly, no significant differences were observed in the ongoing pregnancy and live birth rates, although these data were not available for all women (data for ongoing pregnancy rate were available in 14 cases and for live birth rate in only five patients during the study period).
LH levels during the follicular phase of an IVF cycle has always been a subject of interest. There is a paucity of clear recommendations regarding whether LH levels in the late follicular phase affect the outcomes of IVF cycles. A few studies have demonstrated low pregnancy rates in women undergoing antagonist IVF cycles with very low LH levels on the day of the trigger [5,14]. By contrast, other studies have shown no correlation between trigger day LH levels and the outcome of an IVF cycle [16,17], especially embryo yield and pregnancy rate. The pre-ovulatory increase in E2 leading to a surge in LH levels triggers a series of reactions leading to final oocyte maturation, including loss of gap junctions in the cumulus and oocyte, cumulus expansion, breakdown of germinal vesicles, and resumption of meiosis [20].
Our study had a limited sample size, and hence, we could not demonstrate any significant correlation between serum LH levels on the trigger day and IVF outcome i.e., the number of MII oocytes, grade A embryos, or clinical pregnancy rate. This could be because in all women with LH <1.5 IU/L, the administered trigger was either a dual trigger or HCG, and none of them received only a GnRH agonist as sole trigger. Low levels of LH in antagonist cycles have been shown to be associated with suboptimal oocyte yield and pregnancy rates [13,14]. Keeping in mind these observations, as a departmental policy, we normally administer either HCG or dual trigger in women whose LH levels are below 1.0 IU/L on the day of the trigger. Previous studies showed that if GnRH agonist trigger was used alone in women with low LH levels, the outcome of an IVF cycle is poor [14]. Various strategies have been proposed to overcome LH over suppression and its detrimental effects on the antagonist cycle. One such measure is the addition of low-dose HCG (Inj. HCG 1,500-2,000 IU, IM, along with Inj. GnRH analog). This is safe and prevents the risk of ovarian hyperstimulation, and provides optimal levels of LH for final oocyte maturation [14]. We followed this protocol in all cases with trigger-day LH levels of <1.0 IU/L. Another proposed measure is the use of progesterone-primed ovarian stimulation instead of an antagonist to control LH surge. Progesterone prevents endogenous LH surges while maintaining LH levels >1.8 ng/mL [21,22]. Luo et al. [23], investigated trigger day LH levels in agonist and antagonist cycles and concluded that trigger-day LH levels were more often suppressed in agonist cycles and correlated with pregnancy outcome, but in the antagonist group this correlation was seen only in a subgroup of advanced age women and those with excessively suppressed LH levels. Therefore, it is worth considering the addition of HCG or LH in women with profoundly suppressed LH levels on trigger days [23].
Similarly, a high rate of miscarriage correlated with very low LH levels on trigger days [13]. In our study, there was a trend towards a high number of miscarriages in the group with LH levels >1.5; however, due to a small sample size in this subgroup significant conclusions could not be drawn. Only one miscarriage occurred in women with LH <1.5, whereas the remaining four occurred in women with LH >1.5. In addition, increased miscarriages were observed in women with very low progesterone levels, which might indicate inadequate luteal support in these women. However, this data need to be validated in studies with a larger sample size. Low follicular phase LH levels lead to low estradiol levels, a poor luteal phase, and reduced ability to form a viable conceptus. Previous studies showed that low serum LH levels on the day of the trigger were correlated with an increased risk of miscarriage, although it is difficult to define the threshold below which the risk of miscarriage increases in women undergoing antagonist IVF [13]. Further, no significant differences were found in either group in the trigger day LH levels, the type of trigger administered, and clinical pregnancy rate.
Our study did not find a significant relationship between serum LH levels, MII retrieval rates, and clinical pregnancy rates. The group with low LH levels required slightly longer days of stimulation and higher doses of gonadotropins. Further, women with LH levels >1.5 IU/L on trigger day had a trend for significantly higher number of miscarriages. Future adequately powered studies with larger sample size, and randomized design are needed for more robust conclusions.
Notes
Fig. 1
Distribution of patients by serum luteinizing hormone levels on the day of trigger. LH, luteinizing hormone; CI, confidence intervals.
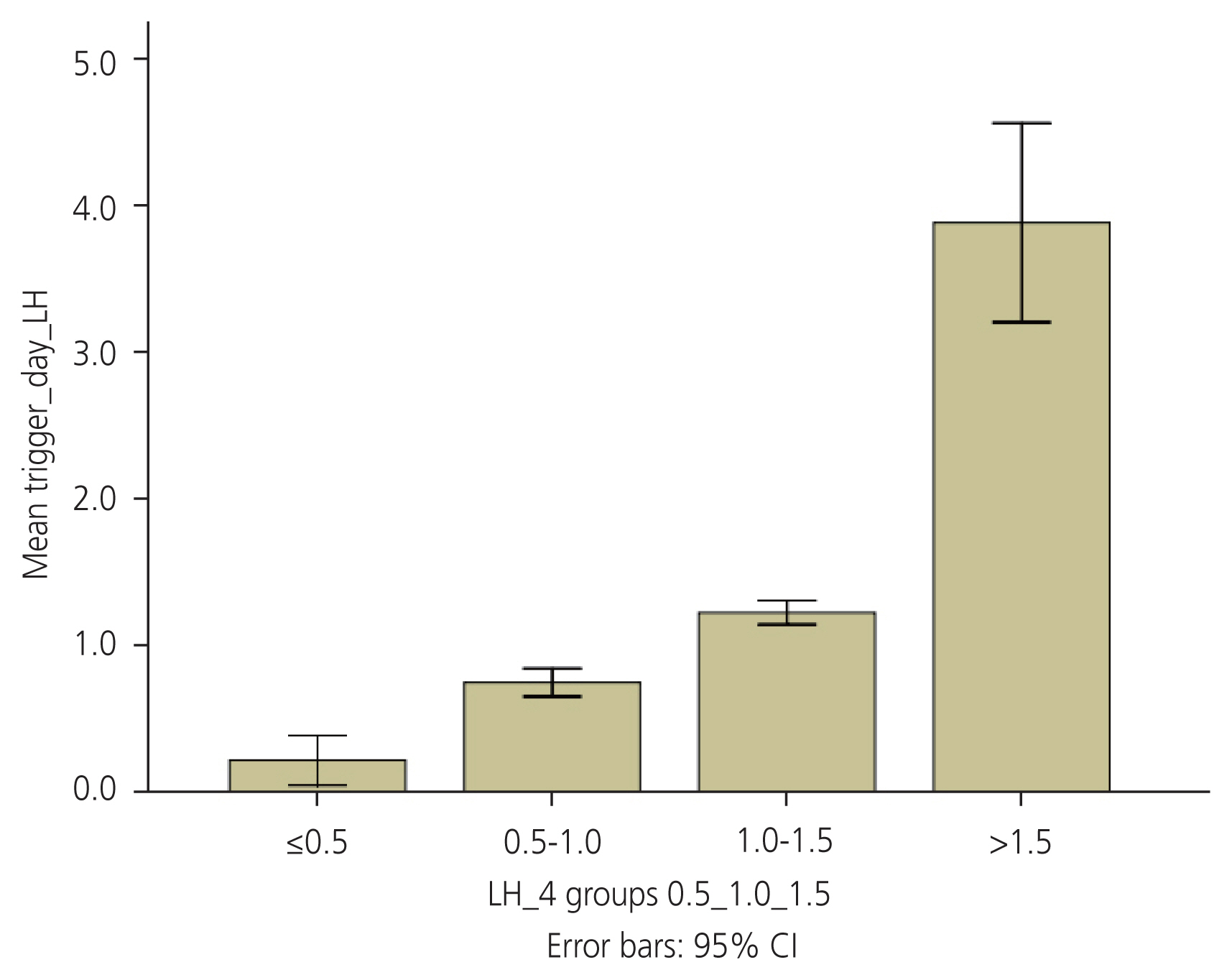
Fig. 2
Relationship of serum luteinizing hormone levels and total dose of gonadotropins (IU). LH, luteinizing hormone; NS, not significant; IU, international units. *Significant difference.
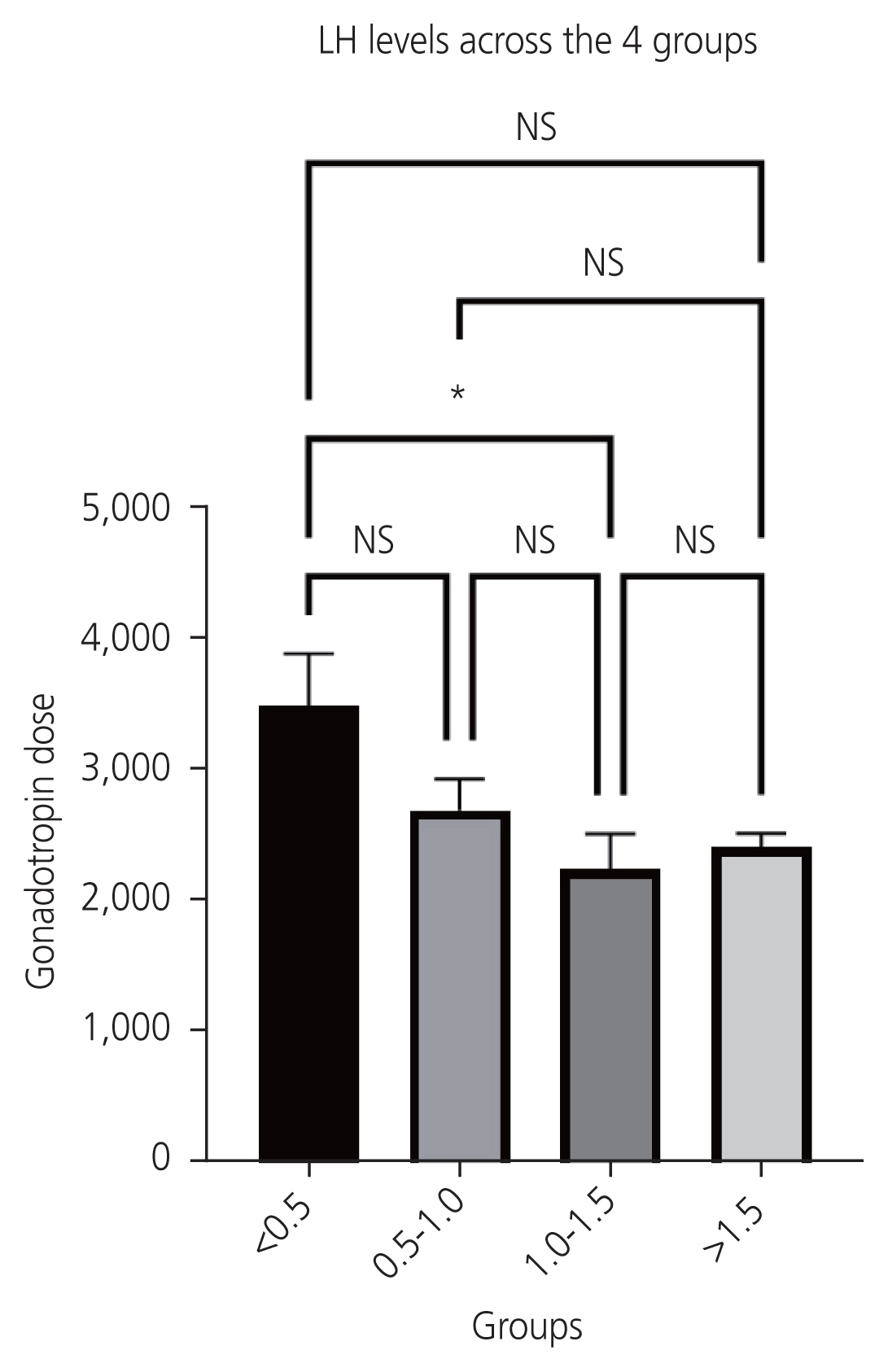
Fig. 3
Relationship between serum luteinizing hormone levels and total days of stimulation. LH, luteinizing hormone; SE, standard error.
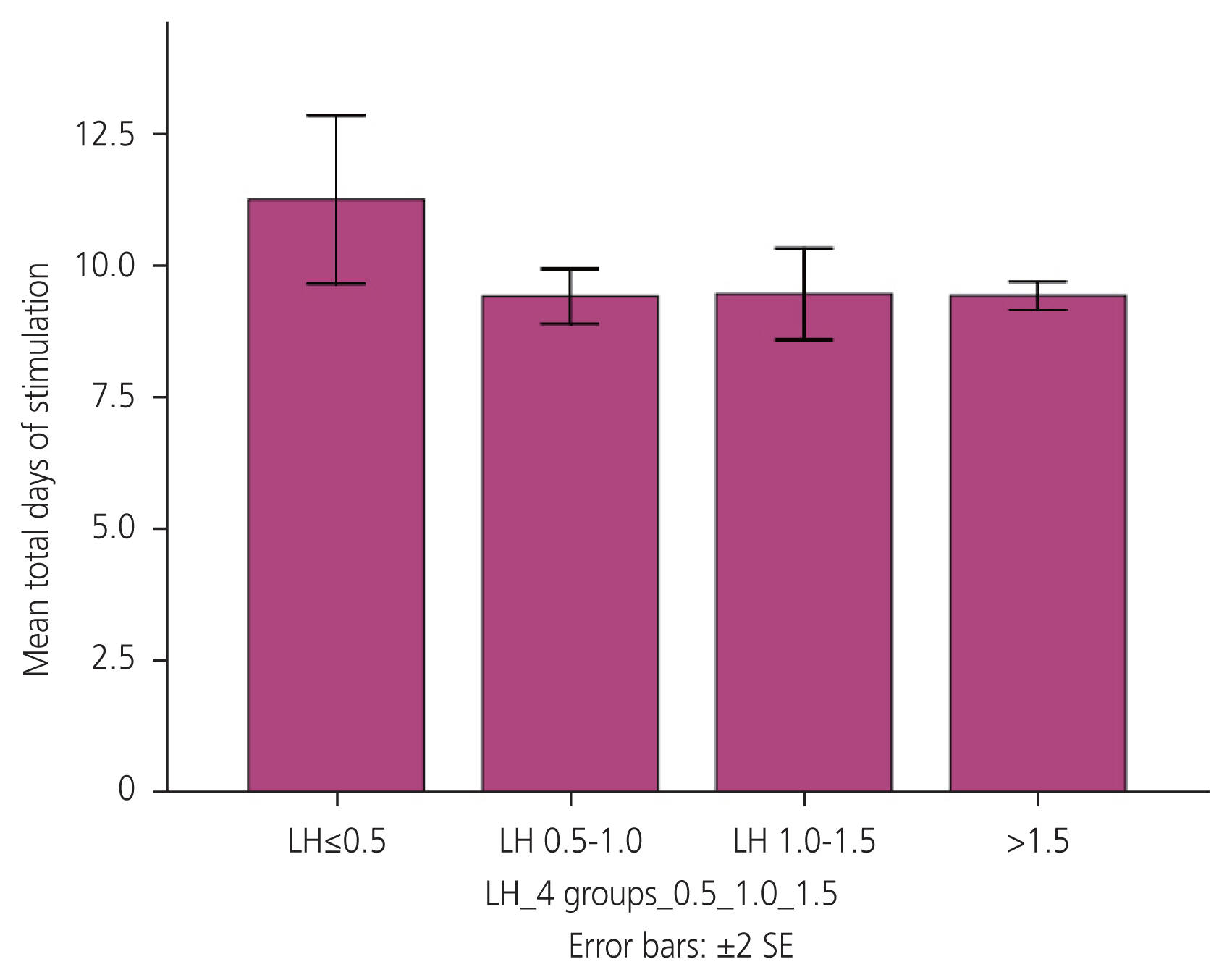
Fig. 4
Oocyte retrieval rare across the four groups. MII, mature oocytes; LH, luteinizing hormone; CI, confidence intervals.
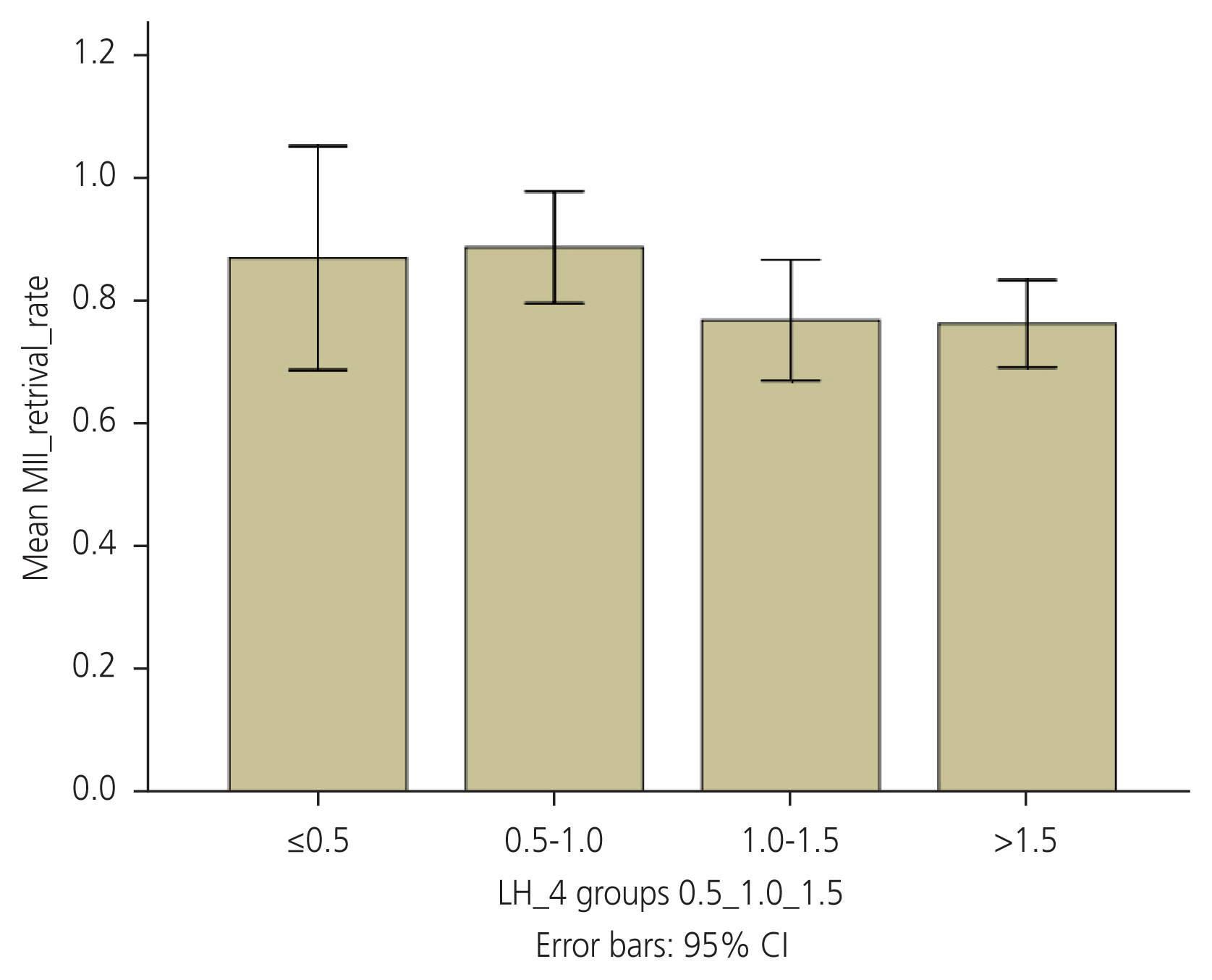
Table 1
Baseline characteristics and details on ovarian stimulation
Table 2
Luteinizing hormonelevels and type of trigger
Table 3
Clinical pregnancy rate
Table 4
Relationship of serum luteinizing hormone levels, type of trigger, and clinical pregnancy rate
References
1. van der Gaast MH, Eijkemans MJC, Van der Net JB, Boer EJ, Burger CW, Van Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online 2016;13:476-80.

2. Blockeel C, Sterrenburg MD, Broekmans FJ, Eijkemans MJ, Smitz J, Devroey P, et al. Follicular phase endocrine characteristics during ovarian stimulation and GnRH antagonist cotreatment for IVF: RCT comparing recFSH initiated on cycle day 2 or 5. J Clin Endocrinol Metab 2011;96:1122-8.



3. Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a cochrane review. Hum Reprod 2002;17:874-85.


4. Halim B, Lubis HP. Dual trigger with gonadotropin-releasing hormone agonist and recombinant human chorionic gonadotropin improves the outcome of intrauterine insemination. Obstet Gynecol Sci 2022;65:207-14.




5. Zhou R, Dong M, Huang L, Zhu X, Wei J, Zhang Q, et al. Association between serum LH levels on hCG trigger day and live birth rate after fresh embryo transfer with GnRH antagonist regimen in different populations. Front Endocrinol (Lausanne) 2023;14:1191827.



6. Benmachiche A, Benbouhedja S, Zoghmar A, Humaidan P. Low LH level on the day of GnRH agonist trigger is associated with reduced ongoing pregnancy and live birth rates and increased early miscarriage rates following IVF/ICSI Tteatment and fresh embryo transfer. Front Endocrinol 2019;10:639.
7. Meyer L, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z, Cholst IN. Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril 2015;104:637-42.


8. Lu X, Hong Q, Sun L, Chen Q, Fu Y, Ai A, et al. Dual trigger for final oocyte maturation improves the oocyte retrieval rate of suboptimal responders to gonadotropin-releasing hormone agonist. Fertil Steril 2016;106:1356-62.


9. Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab 2003;88:4186-92.


10. Weston AM, Zelinski-Wooten MB, Hutchison JS, Stouffer RL, Wolf DP. Developmental potential of embryos produced by in-vitro fertilization from gonadotrophin-releasing hormone antagonist-treated macaques stimulated with recombinant human follicle stimulating hormone alone or in combination with luteinizing hormone. Hum Reprod 1996;11:608-13.


11. Shoham Z, Balen A, Patel A, Jacobs HS. Results of ovulation induction using human menopausal gonadotropin or purified follicle-stimulating hormone in hypogonadotropic hypogonadism patients. Fertil Steril 1991;56:1048-53.


12. Griesinger G, Dawson A, Schultze-Mosgau A, Finas D, Diedrich K, Felberbaum R. Assessment of luteinizing hormone level in the gonadotropin-releasing hormone antagonist protocol. Fertil Steril 2006;85:791-3.


13. Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod 2000;15:1003-8.


14. Propst AM, Hill MJ, Bates GW, Palumbo M, Van Horne AK, Retzloff MG. Low-dose human chorionic gonadotropin may improve in vitro fertilization cycle outcomes in patients with low luteinizing hormone levels after gonadotropin-releasing hormone antagonist administration. Fertil Steril 2011;96:898-904.


15. Kummer N, Benadiva C, Feinn R, Mann J, Nulsen J, Engmann L. Factors that predict the probability of a successful clinical outcome after induction of oocyte maturation with a gonadotropin-releasing hormone agonist. Fertil Steril 2011;96:63-8.


16. Merviel P, Antoine JM, Mathieu E, Millot F, Mandelbaum J, Uzan S. Luteinizing hormone concentrations after gonadotropin-releasing hormone antagonist administration do not influence pregnancy rates in in vitro fertilization-embryo transfer. Fertil Steril 2004;82:119-25.


17. Chen SL, Ye DS, Chen X, Yang XH, Zheng HY, Tang Y, et al. Circulating luteinizing hormone level after triggering oocyte maturation with GnRH agonist may predict oocyte yield in flexible GnRH antagonist protocol. Hum Reprod 2012;27:1351-6.


18. Lin YC, Chang SY, Lan KC, Huang HW, Chang CY, Tsai MY, et al. Human oocyte maturity in vivo determines the outcome of blastocyst development in vitro. J Assist Reprod Genet 2003;20:506-12.



19. Veek LL. An Atlas of human gametes and conceptuses. 1st ed. London: Parthenon; 1999.
20. Orvieto R. Triggering final follicular maturation--hCG, GnRH-agonist or both, when and to whom? J Ovarian Res 2015;8:60.



21. Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril 2015;104:62-70e3.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 618 View
- 79 Download
- Related articles in Obstet Gynecol Sci
-
Effects of super high serum estradiol levels on in vitro fertilization outcome.1991 September;34(9)
Impact of presence of antiphospholipid antibodies on
in vitro fertilization outcome2018 May;61(3)




















